Abstract
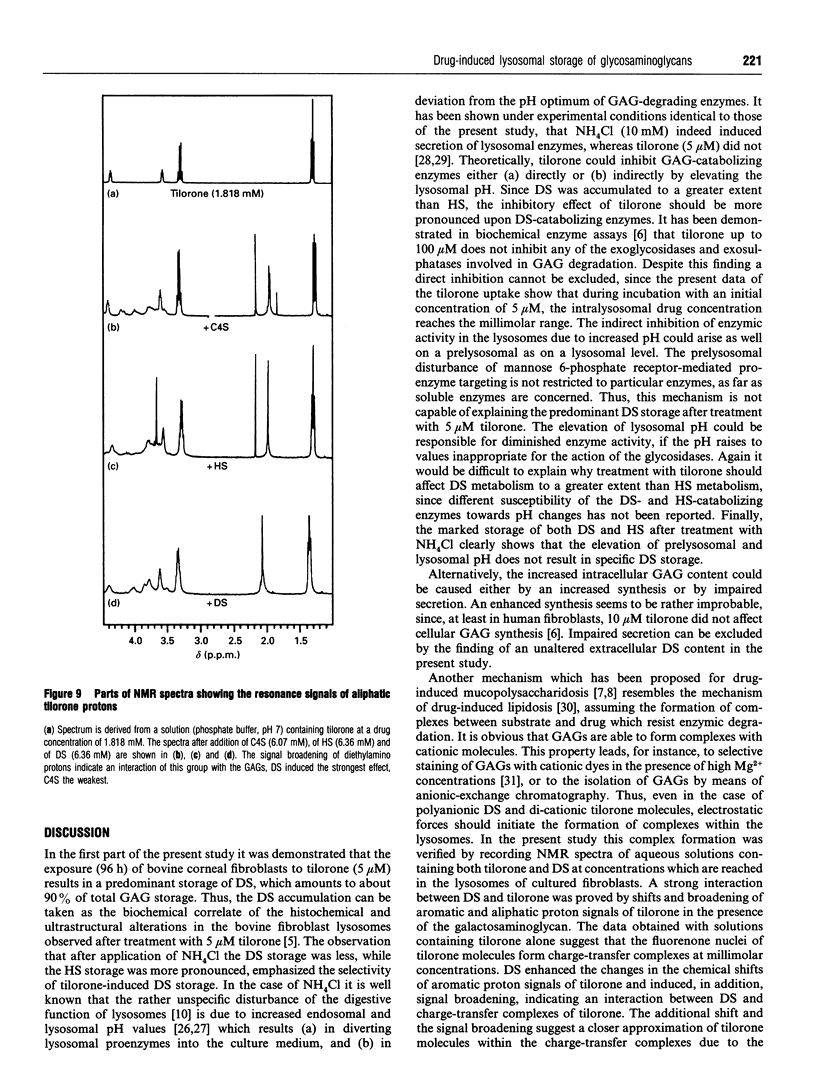
Tilorone (2,7-bis[2-(diethylamino)ethoxy]-fluoren-9-one) and several other bis-basic compounds are known to induce lysosomal glycosaminoglycan (GAG) storage. The responsible pathomechanism has not been elucidated yet. The assumption of an unspecific disturbance of lysosomal proenzyme targeting due to elevation of endosomal pH is opposed by the hypothesis of formation of a complex between tilorone and GAGs within the lysosomes, which renders GAGs indigestible to glycosidases. In cultures of bovine corneal fibroblasts the amounts of intracellular GAGs [dermatan sulphate (DS), heparan sulphate (HS) and chondroitin sulphate (CS)] were quantified. The fibroblasts were exposed to tilorone (5 microM), which was found to be readily taken up by the cells and to be accumulated within acidic compartments to finally achieve millimolar concentrations. Under these conditions the GAG storage is predominantly due to the accumulation of DS; however, the DS secretion into the culture medium was not affected. The HS accumulation was much less pronounced, accounting only for 3% of total GAG storage. Ammonium chloride (10 mM), which is known to diminish lysosomal enzyme activity by interfering with the mannose 6-phosphate receptor-mediated transport, prevents both HS and DS breakdown. By means of NMR spectroscopy it was shown that tilorone itself tends to display a concentration-dependent aggregation which was enhanced in the presence of GAGs. The diethylamino groups of tilorone interact physicochemically with DS, and to a smaller extent with HS, but not with chondroitin 4-sulphate. Thus, the strength of the interaction between tilorone and the different GAGs in vitro correlates with the potency of tilorone to inhibit the breakdown of the individual GAGs in cultured bovine fibroblasts. The results support the hypothesis of a specific interaction between tilorone and particular GAGs, rendering these resistant to enzymic degradation.
Full text
PDF


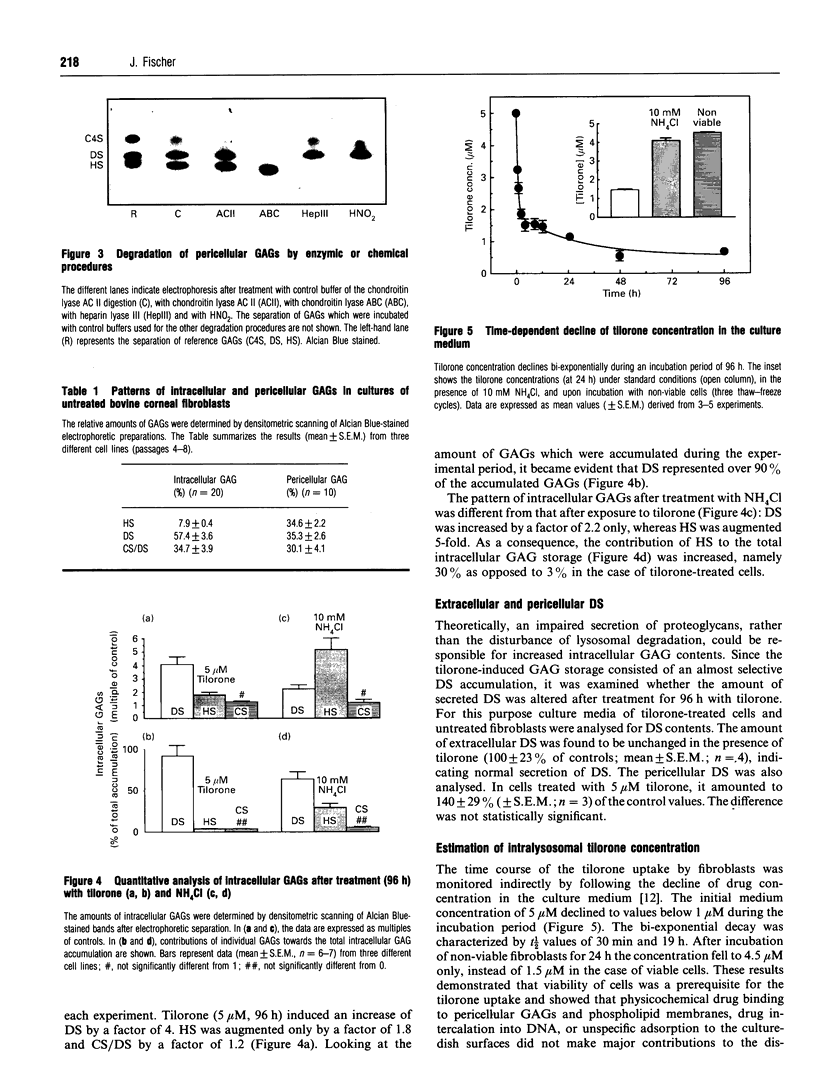
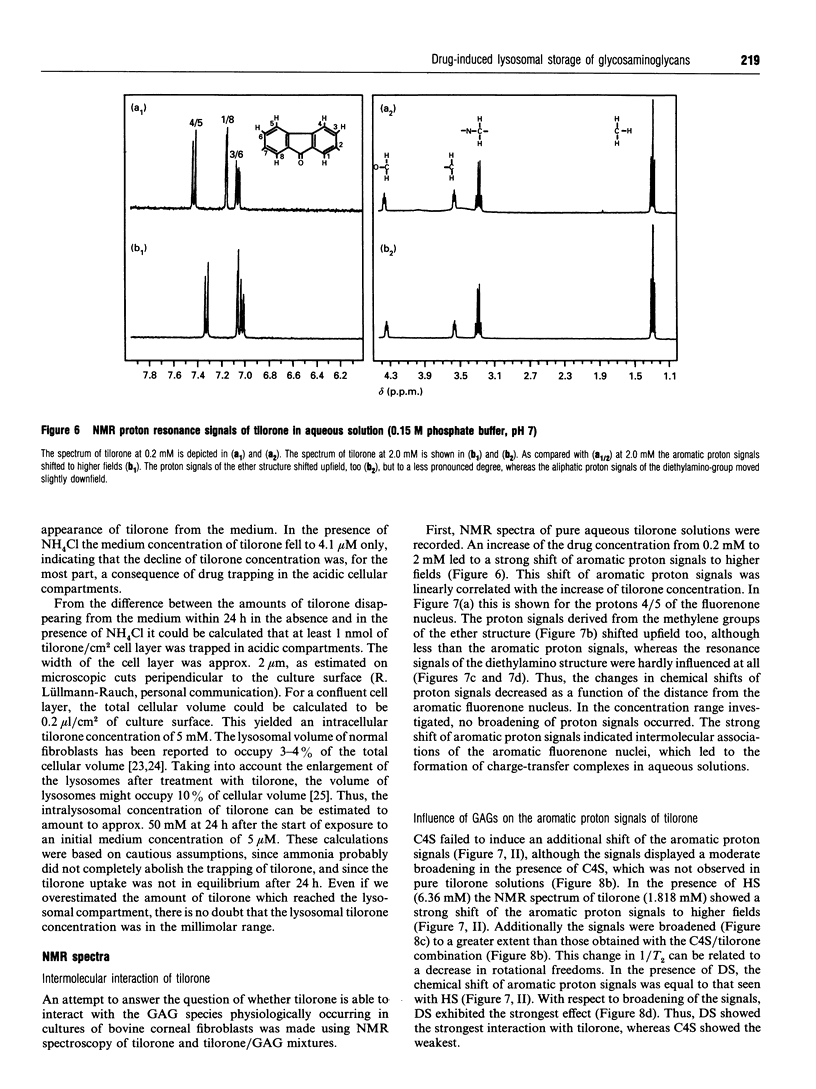
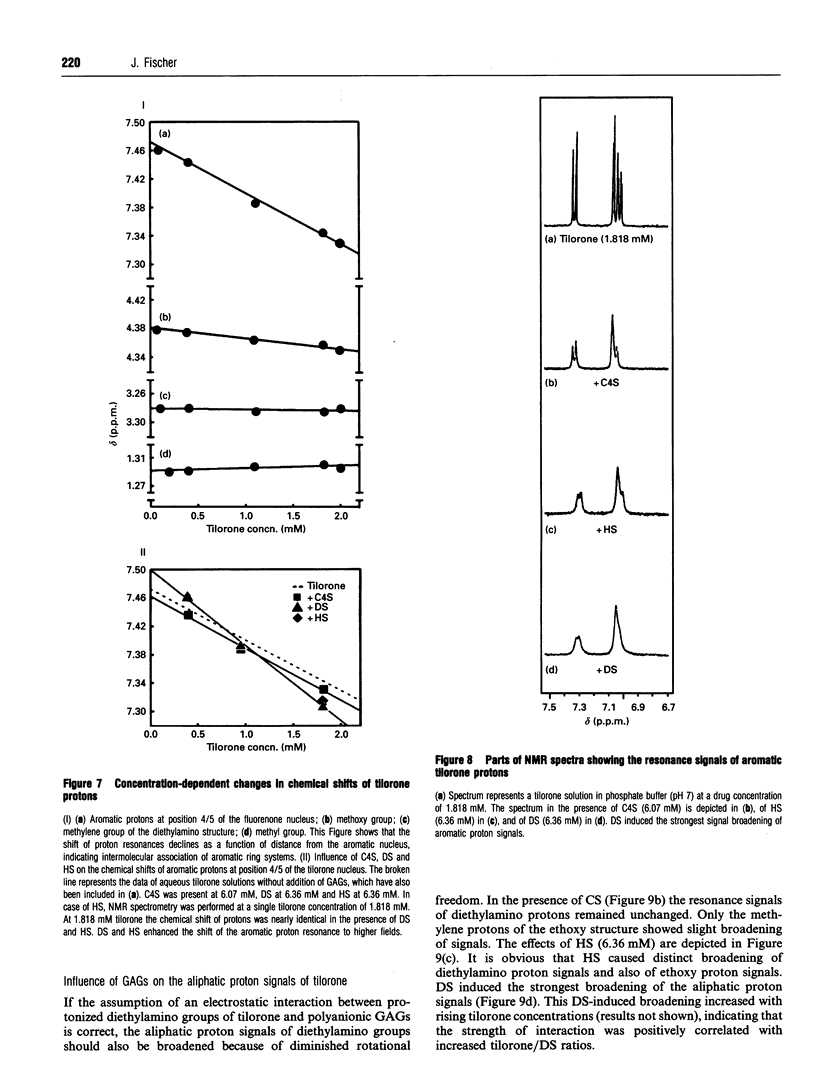

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bleckmann H., Kresse H. Beeinflussung der Glykosaminoglykansynthese von kultivierten Stromazellen aus Rindercorneae durch Variation der Kulturbedingungen. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1979 Jun 15;210(4):291–300. [PubMed] [Google Scholar]
- Bleckmann H., Kresse H. Glycosaminoglycan metabolism of cultured cornea cells derived from bovine and human stroma and from bovine epithelium. Exp Eye Res. 1980 May;30(5):469–479. doi: 10.1016/0014-4835(80)90031-7. [DOI] [PubMed] [Google Scholar]
- Burmester J., Handrock K., Lüllmann-Rauch R. Cultured corneal fibroblasts as a model system for the demonstration of drug-induced mucopolysaccharidosis. Arch Toxicol. 1990;64(4):291–298. doi: 10.1007/BF01972989. [DOI] [PubMed] [Google Scholar]
- Chandra P., Wright G. J. Tilorone hydrochloride: the drug profile. Top Curr Chem. 1977;72:125–148. doi: 10.1007/BFb0048451. [DOI] [PubMed] [Google Scholar]
- Curwen K. D., Smith S. C. Quantitative microanalysis of aortic glycosaminoglycans. Anal Biochem. 1977 May 1;79(1-2):291–301. doi: 10.1016/0003-2697(77)90404-3. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Noriega A., Grubb J. H., Talkad V., Sly W. S. Chloroquine inhibits lysosomal enzyme pinocytosis and enhances lysosomal enzyme secretion by impairing receptor recycling. J Cell Biol. 1980 Jun;85(3):839–852. doi: 10.1083/jcb.85.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grave S., Lüllmann H., Lüllmann-Rauch R., Osterkamp G., Prokopek M. Induction of mucopolysaccharidosis in rats by treatment with immunostimulatory acridine derivatives. Toxicol Appl Pharmacol. 1992 Jun;114(2):215–224. doi: 10.1016/0041-008x(92)90071-y. [DOI] [PubMed] [Google Scholar]
- Gupta D. K., Gieselmann V., Hasilik A., von Figura K. Tilorone acts as a lysosomotropic agent in fibroblasts. Hoppe Seylers Z Physiol Chem. 1984 Aug;365(8):859–866. doi: 10.1515/bchm2.1984.365.2.859. [DOI] [PubMed] [Google Scholar]
- Habuchi H., Yamagata T., Iwata H., Suzuki S. The occurrence of a wide variety of dermatan sulfate-chondroitin sulfate copolymers in fibrous cartilage. J Biol Chem. 1973 Sep 10;248(17):6019–6028. [PubMed] [Google Scholar]
- Hein L., Lüllmann-Rauch R. Mucopolysaccharidosis and lipidosis in rats treated with tilorone analogues. Toxicology. 1989 Oct 2;58(2):145–154. doi: 10.1016/0300-483x(89)90004-8. [DOI] [PubMed] [Google Scholar]
- Hiyama K., Okada S. Crystallization and some properties of chondroitinase from Arthrobacter aurescens. J Biol Chem. 1975 Mar 10;250(5):1824–1828. [PubMed] [Google Scholar]
- Hollemans M., Elferink R. O., De Groot P. G., Strijland A., Tager J. M. Accumulation of weak bases in relation to intralysosomal pH in cultured human skin fibroblasts. Biochim Biophys Acta. 1981 Apr 22;643(1):140–151. doi: 10.1016/0005-2736(81)90226-1. [DOI] [PubMed] [Google Scholar]
- Linker A., Hovingh P. The enzymatic degradation of heparin and heparitin sulfate. I. The fractionation of a crude heparinase from flavobacteria. J Biol Chem. 1965 Oct;240(10):3724–3728. [PubMed] [Google Scholar]
- Lüllmann-Rauch R., Pods R., Von Witzendorff B. Tilorone-induced lysosomal storage of sulphated glycosaminoglycans can be separated from tilorone-induced enhancement of lysosomal enzyme secretion. Biochem Pharmacol. 1995 May 11;49(9):1223–1233. doi: 10.1016/0006-2952(95)00042-x. [DOI] [PubMed] [Google Scholar]
- Lüllmann-Rauch R. Tilorone-induced lysosomal storage mimicking the features of mucopolysaccharidosis and of lipidosis in rat liver. Virchows Arch B Cell Pathol Incl Mol Pathol. 1983;44(3):355–368. doi: 10.1007/BF02890183. [DOI] [PubMed] [Google Scholar]
- Lüllmann-Rauch R., Ziegenhagen M. Drug-induced lysosomal storage of sulfated glycosaminoglycans in cultured bovine and human fibroblasts. Virchows Arch B Cell Pathol Incl Mol Pathol. 1991;60(2):99–104. doi: 10.1007/BF02899533. [DOI] [PubMed] [Google Scholar]
- Lüllmann H., Lüllmann-Rauch R., Wassermann O. Lipidosis induced by amphiphilic cationic drugs. Biochem Pharmacol. 1978;27(8):1103–1108. doi: 10.1016/0006-2952(78)90435-5. [DOI] [PubMed] [Google Scholar]
- MacIntyre A. C., Cutler D. J. Role of lysosomes in hepatic accumulation of chloroquine. J Pharm Sci. 1988 Mar;77(3):196–199. doi: 10.1002/jps.2600770303. [DOI] [PubMed] [Google Scholar]
- Menter J. M., Hurst R. E., West S. S. Thermodynamics of mucopolysaccharide-dye binding. II. Binding constant and cooperativity parameters of acridine orange-dermatan sulfate system. Biopolymers. 1977 Mar;16(3):695–702. doi: 10.1002/bip.1977.360160317. [DOI] [PubMed] [Google Scholar]
- Ohkuma S., Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole B., Ohkuma S. Effect of weak bases on the intralysosomal pH in mouse peritoneal macrophages. J Cell Biol. 1981 Sep;90(3):665–669. doi: 10.1083/jcb.90.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokopek M. The tilorone-induced mucopolysaccharidosis in rats. Biochemical investigations. Biochem Pharmacol. 1991 Nov 6;42(11):2187–2191. doi: 10.1016/0006-2952(91)90355-9. [DOI] [PubMed] [Google Scholar]
- Regelson W. The biological activity of the synthetic polyanion, pyran copolymer (diveema, MVE, 46015) and the heterocyclic bis DEAE fluorenone derivative, tilorone and congeners: clinical and laboratory effects of these agents as modulators of host resistance. Pharmacol Ther. 1981;15(1):1–44. doi: 10.1016/0163-7258(81)90014-0. [DOI] [PubMed] [Google Scholar]
- Saito H., Yamagata T., Suzuki S. Enzymatic methods for the determination of small quantities of isomeric chondroitin sulfates. J Biol Chem. 1968 Apr 10;243(7):1536–1542. [PubMed] [Google Scholar]
- Scott J. E., Chen Y., Brass A. Secondary and tertiary structures involving chondroitin and chondroitin sulphates in solution, investigated by rotary shadowing/electron microscopy and computer simulation. Eur J Biochem. 1992 Oct 15;209(2):675–680. doi: 10.1111/j.1432-1033.1992.tb17335.x. [DOI] [PubMed] [Google Scholar]
- Scott J. E. Collagen--proteoglycan interactions. Localization of proteoglycans in tendon by electron microscopy. Biochem J. 1980 Jun 1;187(3):887–891. doi: 10.1042/bj1870887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. E., Heatley F. Detection of secondary structure in glycosaminoglycans via the H n.m.r. signal of the acetamido NH group. Biochem J. 1982 Oct 1;207(1):139–144. doi: 10.1042/bj2070139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. E., Heatley F., Jones M. N., Wilkinson A., Olavesen A. H. Secondary structure of chondroitin sulphate in dimethyl sulphoxide. Eur J Biochem. 1983 Feb 15;130(3):491–495. doi: 10.1111/j.1432-1033.1983.tb07177.x. [DOI] [PubMed] [Google Scholar]
- Scott J. E. Supramolecular organization of extracellular matrix glycosaminoglycans, in vitro and in the tissues. FASEB J. 1992 Jun;6(9):2639–2645. [PubMed] [Google Scholar]
- Seglen P. O. Inhibitors of lysosomal function. Methods Enzymol. 1983;96:737–764. doi: 10.1016/s0076-6879(83)96063-9. [DOI] [PubMed] [Google Scholar]
- Shively J. E., Conrad H. E. Formation of anhydrosugars in the chemical depolymerization of heparin. Biochemistry. 1976 Sep 7;15(18):3932–3942. doi: 10.1021/bi00663a005. [DOI] [PubMed] [Google Scholar]
- Stramm L. E., Li W., Aguirre G. D., Rockey J. H. Glycosaminoglycan synthesis and secretion by bovine retinal capillary pericytes in culture. Exp Eye Res. 1987 Jan;44(1):17–28. doi: 10.1016/s0014-4835(87)80021-0. [DOI] [PubMed] [Google Scholar]
- Wessler E. Analytical and preparative separation of acidic glycosaminoglycans by electrophoresis in barium acetate. Anal Biochem. 1968 Dec;26(3):439–444. doi: 10.1016/0003-2697(68)90205-4. [DOI] [PubMed] [Google Scholar]
- Wibo M., Poole B. Protein degradation in cultured cells. II. The uptake of chloroquine by rat fibroblasts and the inhibition of cellular protein degradation and cathepsin B1. J Cell Biol. 1974 Nov;63(2 Pt 1):430–440. doi: 10.1083/jcb.63.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Duve C., de Barsy T., Poole B., Trouet A., Tulkens P., Van Hoof F. Commentary. Lysosomotropic agents. Biochem Pharmacol. 1974 Sep 15;23(18):2495–2531. doi: 10.1016/0006-2952(74)90174-9. [DOI] [PubMed] [Google Scholar]