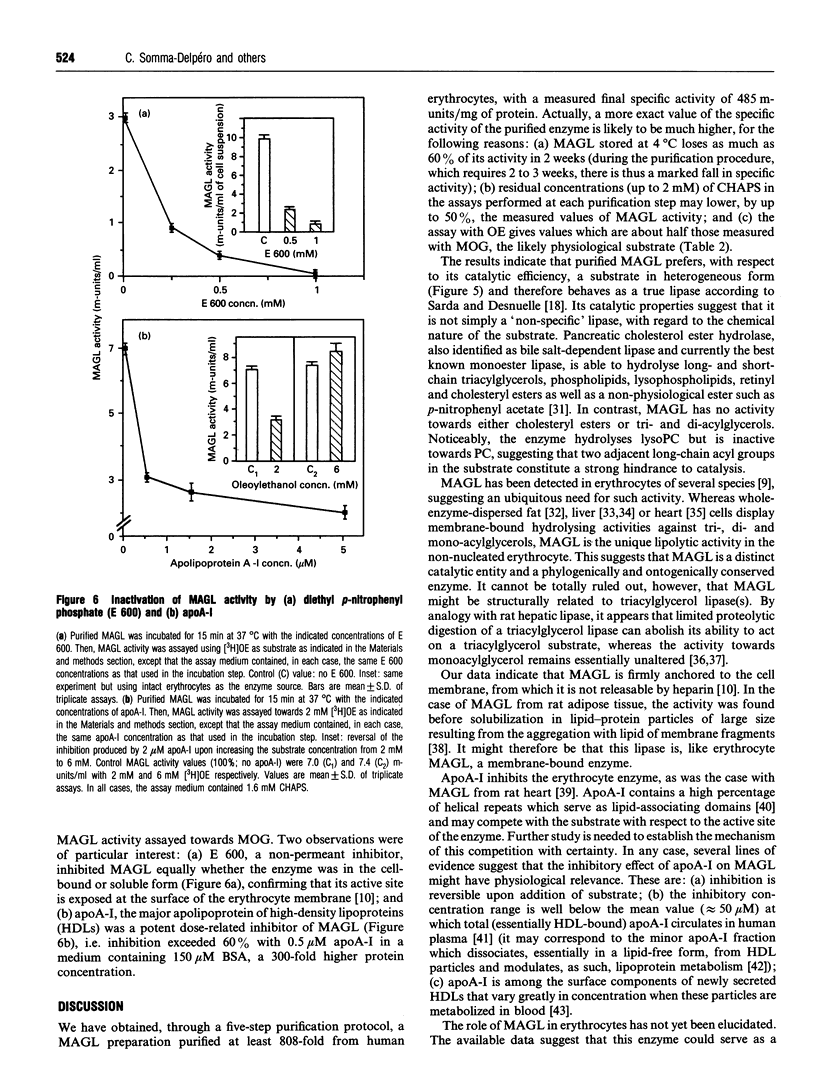
Abstract
A membrane-bound monoacylglycerol lipase (MAGL) activity, previously demonstrated in intact human erythrocytes [Boyer, Somma, Vérine, L'Hôte, Finidori, Merger and Arnaud (1981) J. Clin. Endocrinol. Metab. 53, 143-148], has now been purified to apparent homogeneity by a five-step procedure involving solubilization in CHAPS and sequential chromatographies on Sephacryl S-400, DEAE-Trisacryl, Zn(2+)-chelating Sepharose and Superose 12 columns. The purified protein has a molecular mass of 68 +/- 2 kDa, as determined by SDS/PAGE and gel filtration, suggesting that the enzyme behaves as a monomer. The concentration-dependence of MAGL activity with monooleoylglycerol, the preferred substrate showed kinetics typical of an interfacial lipolytic enzyme displaying optimal activity on emulsified substrate particles; apparent Km values were 0.27 mM and 0.49 mM for the sn-1(3)- and sn-2-isomers respectively. MAGL had no, or negligible, activity towards tri-oleoylglycerol, di-oleoylglycerol, oleoylcholesterol, oleoyl-CoA and phosphatidylcholine; it was inhibited by di-isopropylfluorophosphate, PMSF and diethyl p-nitrophenyl phosphate, suggesting that MAGL is a serine hydrolase. MAGL activity was not modified by bile salt or apolipoprotein C-II, whereas a dose-dependent inhibition was observed with apolipoprotein A-I.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnaud J., Boyer J. Hydrolysis and uptake of an aliphatic fatty ester by whole isolated fat cells. Biochim Biophys Acta. 1977 Mar 25;486(3):462–469. doi: 10.1016/0005-2760(77)90096-0. [DOI] [PubMed] [Google Scholar]
- Arnaud J., Boyer J. Identification of an acylcholesterol lipase activity in human adipose tissue. Biochim Biophys Acta. 1974 Jan 23;337(1):165–168. doi: 10.1016/0005-2760(74)90052-6. [DOI] [PubMed] [Google Scholar]
- Arnaud J., Boyer J. Lipolytic activity of whole isolated liver cells in aqueous suspension. Biochim Biophys Acta. 1976 Mar 26;424(3):460–468. doi: 10.1016/0005-2760(76)90035-7. [DOI] [PubMed] [Google Scholar]
- Arnaud J., Nobili O., Boyer J. Characterization of a monoester lipase active as membrane-bound enzyme in rat erythrocytes. FEBS Lett. 1979 Mar 1;99(1):43–46. doi: 10.1016/0014-5793(79)80244-6. [DOI] [PubMed] [Google Scholar]
- Arnaud J., Nobili O., Boyer J. Characterization of a monoester lipase active as membrane-bound enzyme in rat erythrocytes. FEBS Lett. 1979 Mar 1;99(1):43–46. doi: 10.1016/0014-5793(79)80244-6. [DOI] [PubMed] [Google Scholar]
- Arvidsson E. O., Belfrage P. Monoglyceride-protein interaction. The binding of monoolein to native human serum albumin. Acta Chem Scand. 1969;23(1):232–236. doi: 10.3891/acta.chem.scand.23-0232. [DOI] [PubMed] [Google Scholar]
- Benkirane M., Meignen J. M., Mercier L., Boyer J., Verine A. Lipoprotein lipase in rat heart--III. Effects of sex steroid hormones on tri-, di- and monoacylglycerol lipase activities in post-heparin effluents. Comp Biochem Physiol B. 1989;94(1):27–30. doi: 10.1016/0305-0491(89)90005-9. [DOI] [PubMed] [Google Scholar]
- Berglund L., Khoo J. C., Jensen D., Steinberg D. Resolution of hormone-sensitive triglyceride/diglyceride lipase from monoglyceride lipase of chicken adipose tissue. J Biol Chem. 1980 Jun 10;255(11):5420–5428. [PubMed] [Google Scholar]
- Biale Y., Gorin E., Shafrir E. Characterization of tissue lipolytic and esterolytic activities cleaving full and partial glycerides. Biochim Biophys Acta. 1968 Jan 10;152(1):28–32. doi: 10.1016/0005-2760(68)90005-2. [DOI] [PubMed] [Google Scholar]
- Boyer J., Somma C., Vérine A., L'Hôte C., Finidori J., Merger C., Arnaud J. Human erythrocyte monoester lipase: characterization and radiochemical assay of the cell-bound enzyme in normal subjects. J Clin Endocrinol Metab. 1981 Jul;53(1):143–148. doi: 10.1210/jcem-53-1-143. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bry K., Andersson L. C., Kuusi T., Kinnunen P. K. Monoacylglycerol hydrolase in human platelets. Biochim Biophys Acta. 1979 Oct 26;575(1):121–127. doi: 10.1016/0005-2760(79)90137-1. [DOI] [PubMed] [Google Scholar]
- CARROLL K. K. Separation of lipid classes by chromatography on Florisil. J Lipid Res. 1961 Apr;2:135–141. [PubMed] [Google Scholar]
- Cohen J., Somma-Delpero C., Verine A., Codaccioni J. L., Boyer J. Increased monoester lipase activity in red blood cells during hyperthyroidism. J Endocrinol. 1986 Mar;108(3):357–359. doi: 10.1677/joe.0.1080357. [DOI] [PubMed] [Google Scholar]
- De Jong B. J., Hülsmann W. C. Monoacylglycerol hydrolase activity of isolated rat small intestinal epithelial cells. Biochim Biophys Acta. 1978 Jan 27;528(1):36–46. doi: 10.1016/0005-2760(78)90050-4. [DOI] [PubMed] [Google Scholar]
- Delpéro C., Gastaldi M., Vérine A., Campistron M., Boyer J. Increased monoester lipase activity of red blood cells in alcoholism. Alcohol Clin Exp Res. 1986 Dec;10(6):602–605. doi: 10.1111/j.1530-0277.1986.tb05152.x. [DOI] [PubMed] [Google Scholar]
- Eisenberg S. High density lipoprotein metabolism. J Lipid Res. 1984 Oct;25(10):1017–1058. [PubMed] [Google Scholar]
- Gastaut J. A., Vérine A., Crcassonne Y., Boyer J. Monoester lipase activity in the red cells of patients with various blood disorders. Br J Haematol. 1982 Sep;52(1):127–130. doi: 10.1111/j.1365-2141.1982.tb03869.x. [DOI] [PubMed] [Google Scholar]
- Giudicelli H., Boyer J. Effects of glycerol on human adipose tissue triglyceride lipase activity. J Lipid Res. 1973 Sep;14(5):592–595. [PubMed] [Google Scholar]
- Hee-Cheong M., Severson D. L. Properties of monoacylglycerol lipase in rabbit aorta. Lipids. 1987 Dec;22(12):999–1004. doi: 10.1007/BF02536439. [DOI] [PubMed] [Google Scholar]
- Jensen G. L., Daggy B., Bensadoun A. Triacylglycerol lipase, monoacylglycerol lipase and phospholipase activities of highly purified rat hepatic lipase. Biochim Biophys Acta. 1982 Mar 12;710(3):464–470. doi: 10.1016/0005-2760(82)90130-8. [DOI] [PubMed] [Google Scholar]
- Kuusi T., Bry K., Nikkilä E. A., Kinnunen P. K. Modification of the substrate specificity of rat hepatic lipase by collagenase treatment. Med Biol. 1979 Jun;57(3):192–195. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehner R., Kuksis A. Purification of an acyl-CoA hydrolase from rat intestinal microsomes. A candidate acyl-enzyme intermediate in glycerolipid acylation. J Biol Chem. 1993 Nov 25;268(33):24726–24733. [PubMed] [Google Scholar]
- Liang H. Q., Rye K. A., Barter P. J. Dissociation of lipid-free apolipoprotein A-I from high density lipoproteins. J Lipid Res. 1994 Jul;35(7):1187–1199. [PubMed] [Google Scholar]
- Lombardo D., Fauvel J., Guy O. Studies on the substrate specificity of a carboxyl ester hydrolase from human pancreatic juice. I. Action on carboxyl esters, glycerides and phospholipids. Biochim Biophys Acta. 1980 Jan 11;611(1):136–146. doi: 10.1016/0005-2744(80)90049-2. [DOI] [PubMed] [Google Scholar]
- MORRISON W. R., SMITH L. M. PREPARATION OF FATTY ACID METHYL ESTERS AND DIMETHYLACETALS FROM LIPIDS WITH BORON FLUORIDE--METHANOL. J Lipid Res. 1964 Oct;5:600–608. [PubMed] [Google Scholar]
- Marteau C., Quibel J. R., Le Petit-Thèvenin J., Boyer J., Gérolami A. Lipolytic activities of freshly isolated rat liver parenchymal cells. Life Sci. 1988;42(5):533–538. doi: 10.1016/0024-3205(88)90094-x. [DOI] [PubMed] [Google Scholar]
- Nilsson-Ehle P., Belfrage P. A monoglyceride hydrolyzing enzyme in human postheparin plasma. Biochim Biophys Acta. 1972 May 23;270(1):60–64. doi: 10.1016/0005-2760(72)90177-4. [DOI] [PubMed] [Google Scholar]
- Patsch J. R., Prasad S., Gotto A. M., Jr, Bengtsson-Olivecrona G. Postprandial lipemia. A key for the conversion of high density lipoprotein2 into high density lipoprotein3 by hepatic lipase. J Clin Invest. 1984 Dec;74(6):2017–2023. doi: 10.1172/JCI111624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters T., Jr Serum albumin. Adv Protein Chem. 1985;37:161–245. doi: 10.1016/s0065-3233(08)60065-0. [DOI] [PubMed] [Google Scholar]
- Poensgen J. Apolipoprotein C-1 inhibits the hydrolysis by phospholipase A2 of phospholipids in liposomes and cell membranes. Biochim Biophys Acta. 1990 Feb 6;1042(2):188–192. doi: 10.1016/0005-2760(90)90006-j. [DOI] [PubMed] [Google Scholar]
- SARDA L., DESNUELLE P. Action de la lipase pancréatique sur les esters en émulsion. Biochim Biophys Acta. 1958 Dec;30(3):513–521. doi: 10.1016/0006-3002(58)90097-0. [DOI] [PubMed] [Google Scholar]
- Schulthess G., Lipka G., Compassi S., Boffelli D., Weber F. E., Paltauf F., Hauser H. Absorption of monoacylglycerols by small intestinal brush border membrane. Biochemistry. 1994 Apr 19;33(15):4500–4508. doi: 10.1021/bi00181a009. [DOI] [PubMed] [Google Scholar]
- Segrest J. P., Jones M. K., De Loof H., Brouillette C. G., Venkatachalapathi Y. V., Anantharamaiah G. M. The amphipathic helix in the exchangeable apolipoproteins: a review of secondary structure and function. J Lipid Res. 1992 Feb;33(2):141–166. [PubMed] [Google Scholar]
- Serdarevich B., Carroll K. K. Synthesis and characterization of 1- and 2-monoglycerides of anteiso fatty acids. J Lipid Res. 1966 Mar;7(2):277–284. [PubMed] [Google Scholar]
- Somma-Delpero C., Meignen J. M., Jacquème B., Serment H., Boyer J. Increased monoester lipase activity in red blood cells in late pregnancy. Acta Haematol. 1985;73(2):120–121. doi: 10.1159/000206298. [DOI] [PubMed] [Google Scholar]
- Spector A. A., Soboroff J. M. Long chain fatty acid methyl ester hydrolase activity in mammalian cells. Lipids. 1972 Mar;7(3):186–190. doi: 10.1007/BF02533061. [DOI] [PubMed] [Google Scholar]
- Tornqvist H., Belfrage P. Purification and some properties of a monoacylglycerol-hydrolyzing enzyme of rat adipose tissue. J Biol Chem. 1976 Feb 10;251(3):813–819. [PubMed] [Google Scholar]
- Tornqvist H., Nilsson-Ehle P., Belfrage P. Enzymes catalyzing the hydrolysis of long-chain monoacyglycerols in rat adipose tissue. Biochim Biophys Acta. 1978 Sep 28;530(3):474–486. doi: 10.1016/0005-2760(78)90167-4. [DOI] [PubMed] [Google Scholar]
- Verger R. Enzyme kinetics of lipolysis. Methods Enzymol. 1980;64:340–392. doi: 10.1016/s0076-6879(80)64016-6. [DOI] [PubMed] [Google Scholar]
- Verine A., Boyer J. Lipases operative at the fat cell surface: attempt at an integrated approach. Cell Biochem Funct. 1987 Jul;5(3):175–181. doi: 10.1002/cbf.290050304. [DOI] [PubMed] [Google Scholar]