Abstract
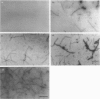
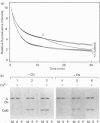
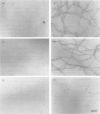
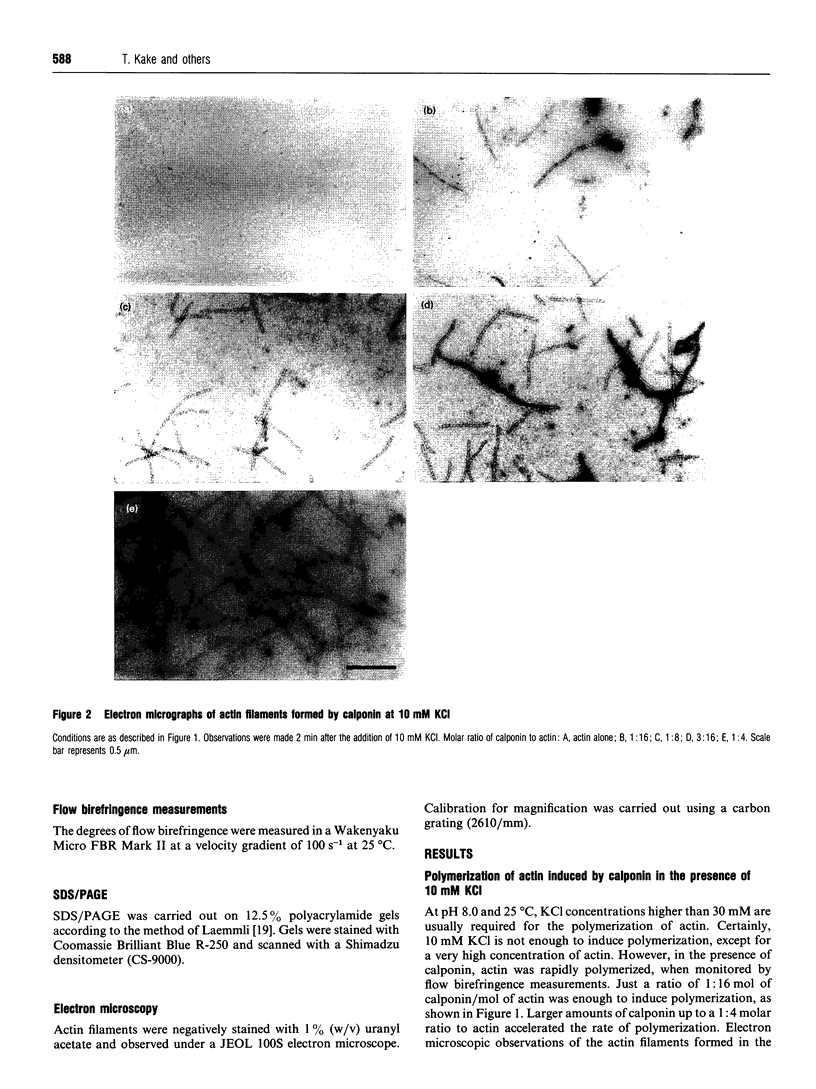
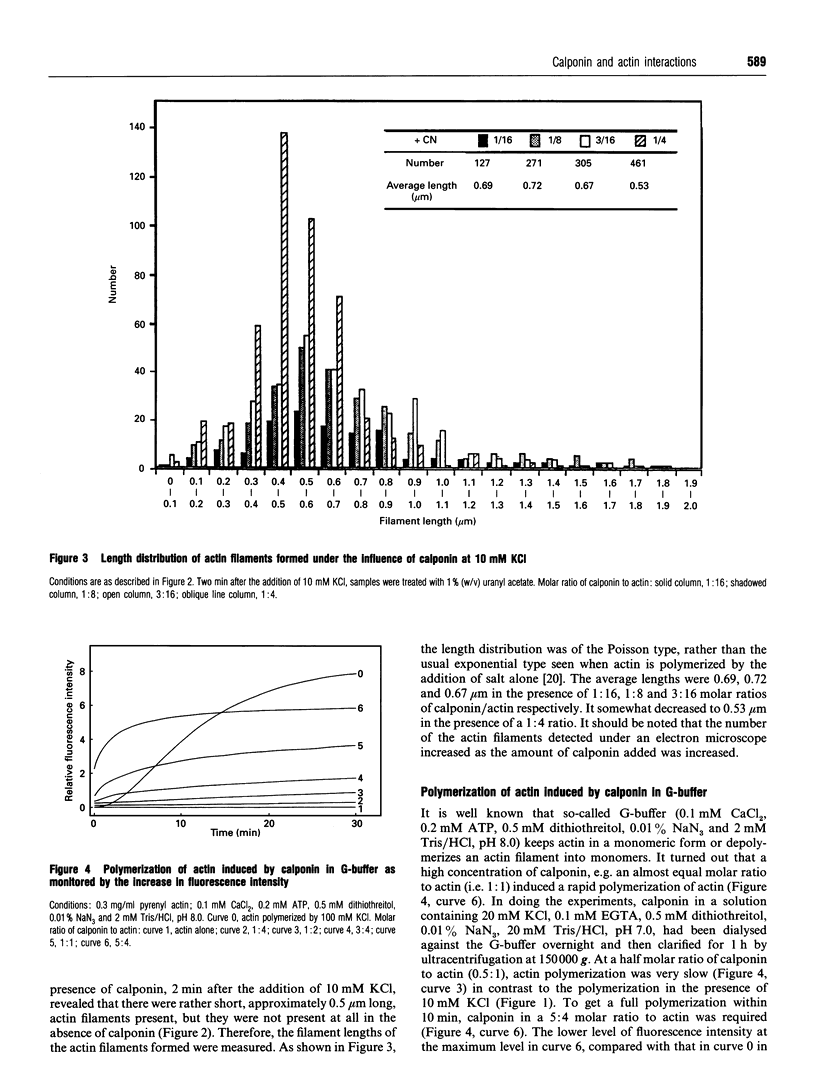
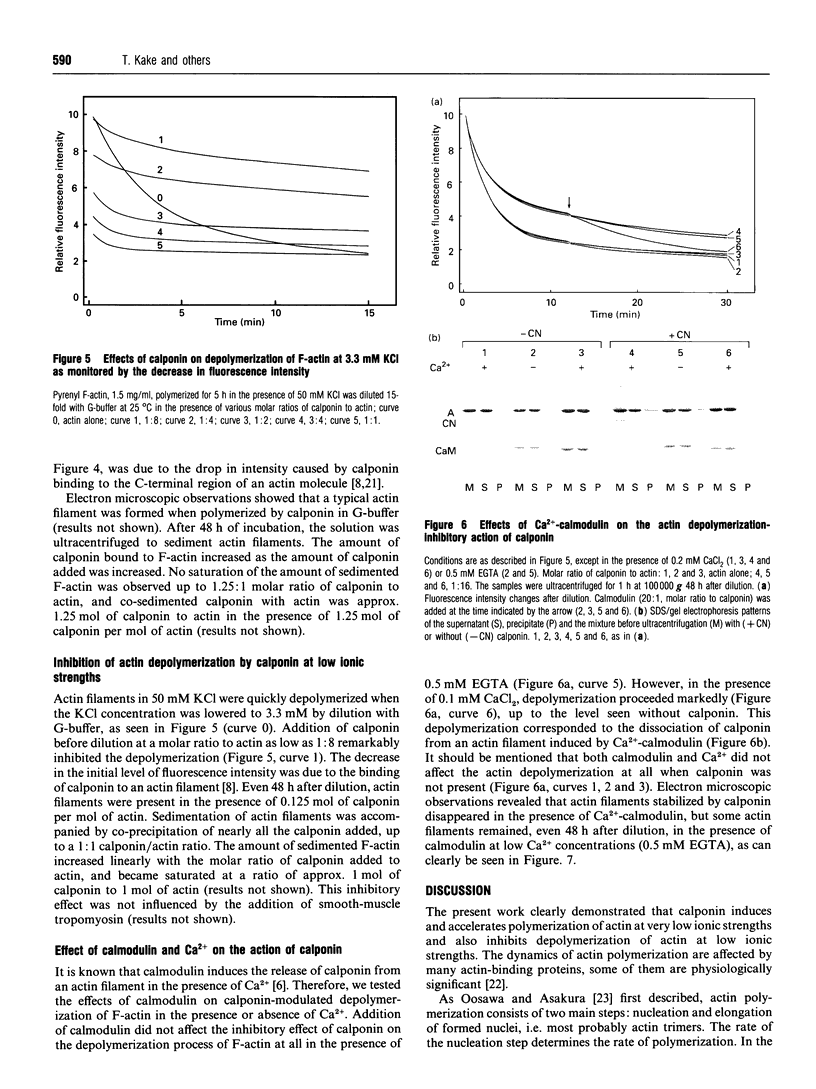
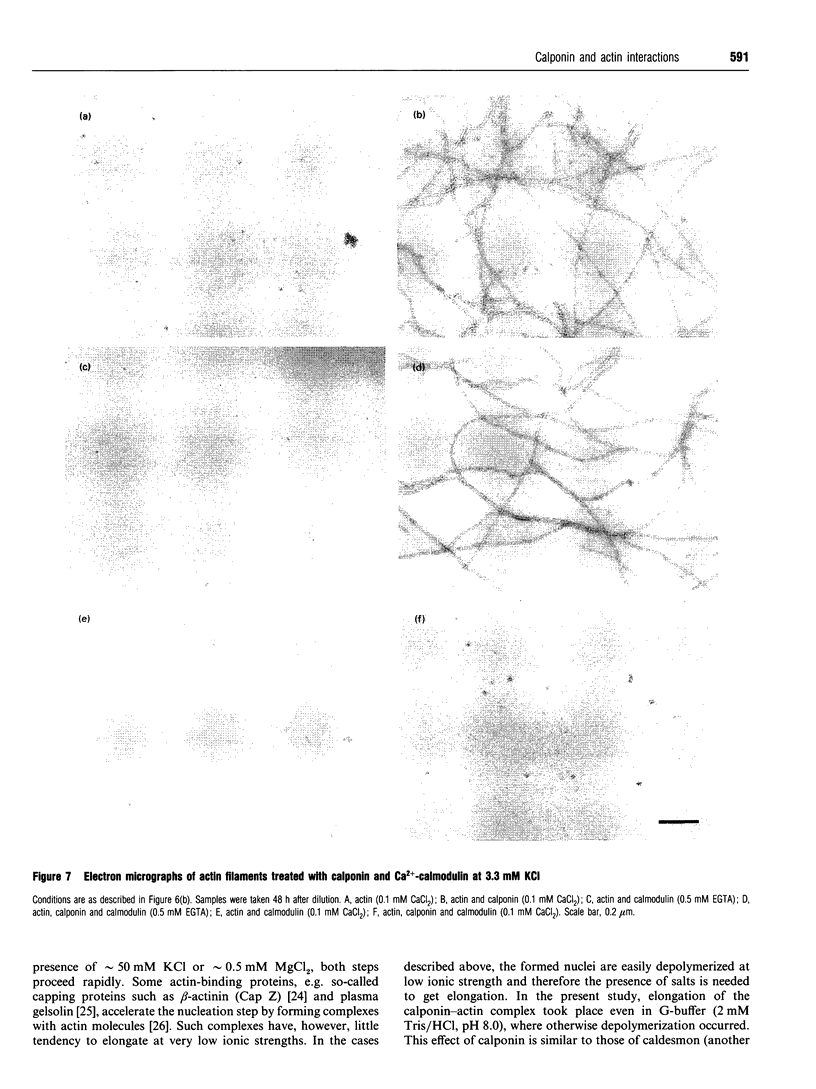
Calponin from chicken gizzard induced polymerization of actin in the presence of 10 mM KCl. Only 2 min after the addition of KCl in the presence of a 0.0625-0.25:1 molar ratio of calponin to actin, a Poisson-type length distribution (with an average length of approx. 0.7 micron) was observed with formed actin filaments. This result suggests that calponin-actin complexes served as nuclei for rapid elongation. Calponin caused a rapid polymerization of actin even in G-buffer (2 mM Tris/HCl, pH 8.0) which is usually used for depolymerization of actin filaments. Binding of calponin at a level of up to 1.25 mol per mol of actin was observed in the actin filaments formed in the presence of calponin at very low ionic strengths. When actin filaments were exposed to 3.3 mM KCl, by dilution with G-buffer, a rapid depolymerization occurred. Addition of calponin greatly retarded the depolymerization process and, in the presence of an equimolar ratio of calponin to actin, depolymerization hardly occurred. In the presence of calmodulin, this inhibitory effect on depolymerization was reversed by Ca2+, releasing calponin from actin filaments.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe M., Takahashi K., Hiwada K. Effect of calponin on actin-activated myosin ATPase activity. J Biochem. 1990 Nov;108(5):835–838. doi: 10.1093/oxfordjournals.jbchem.a123289. [DOI] [PubMed] [Google Scholar]
- Applegate D., Feng W., Green R. S., Taubman M. B. Cloning and expression of a novel acidic calponin isoform from rat aortic vascular smooth muscle. J Biol Chem. 1994 Apr 8;269(14):10683–10690. [PubMed] [Google Scholar]
- Bonet-Kerrache A., Mornet D. Importance of the C-terminal part of actin in interactions with calponin. Biochem Biophys Res Commun. 1995 Jan 5;206(1):127–132. doi: 10.1006/bbrc.1995.1018. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Pollard T. D. Effect of capping protein on the kinetics of actin polymerization. Biochemistry. 1985 Jan 29;24(3):793–799. doi: 10.1021/bi00324a039. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Walker S. B., Pollard T. D. Pyrene actin: documentation of the validity of a sensitive assay for actin polymerization. J Muscle Res Cell Motil. 1983 Apr;4(2):253–262. doi: 10.1007/BF00712034. [DOI] [PubMed] [Google Scholar]
- Fujii T., Oomatsuzawa A., Kuzumaki N., Kondo Y. Calcium-dependent regulation of smooth muscle calponin by S100. J Biochem. 1994 Jul;116(1):121–127. doi: 10.1093/oxfordjournals.jbchem.a124484. [DOI] [PubMed] [Google Scholar]
- Gałazkiewicz B., Belagyi J., Dabrowska R. The effect of caldesmon on assembly and dynamic properties of actin. Eur J Biochem. 1989 May 15;181(3):607–614. doi: 10.1111/j.1432-1033.1989.tb14767.x. [DOI] [PubMed] [Google Scholar]
- Gałazkiewicz B., Mossakowska M., Osińska H., Dabrowska R. Polymerization of G-actin by caldesmon. FEBS Lett. 1985 May 6;184(1):144–149. doi: 10.1016/0014-5793(85)80671-2. [DOI] [PubMed] [Google Scholar]
- Houk T. W., Jr, Ue K. The measurement of actin concentration in solution: a comparison of methods. Anal Biochem. 1974 Nov;62(1):66–74. doi: 10.1016/0003-2697(74)90367-4. [DOI] [PubMed] [Google Scholar]
- Kawamura M., Maruyama K. Electron microscopic particle length of F-actin polymerized in vitro. J Biochem. 1970 Mar;67(3):437–457. doi: 10.1093/oxfordjournals.jbchem.a129267. [DOI] [PubMed] [Google Scholar]
- Kawamura M., Maruyama K. Length distribution of F-actin transformed from Mg-polymer. Biochim Biophys Acta. 1972 May 25;267(2):422–434. doi: 10.1016/0005-2728(72)90129-6. [DOI] [PubMed] [Google Scholar]
- Klee C. B. Conformational transition accompanying the binding of Ca2+ to the protein activator of 3',5'-cyclic adenosine monophosphate phosphodiesterase. Biochemistry. 1977 Mar 8;16(5):1017–1024. doi: 10.1021/bi00624a033. [DOI] [PubMed] [Google Scholar]
- Kouyama T., Mihashi K. Fluorimetry study of N-(1-pyrenyl)iodoacetamide-labelled F-actin. Local structural change of actin protomer both on polymerization and on binding of heavy meromyosin. Eur J Biochem. 1981;114(1):33–38. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehman W. Calponin and the composition of smooth muscle thin filaments. J Muscle Res Cell Motil. 1991 Jun;12(3):221–224. doi: 10.1007/BF01745110. [DOI] [PubMed] [Google Scholar]
- Makuch R., Birukov K., Shirinsky V., Dabrowska R. Functional interrelationship between calponin and caldesmon. Biochem J. 1991 Nov 15;280(Pt 1):33–38. doi: 10.1042/bj2800033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama K., Kimura S., Ishi T., Kuroda M., Ohashi K. beta-actinin, a regulatory protein of muscle. Purification, characterization and function. J Biochem. 1977 Jan;81(1):215–232. doi: 10.1093/oxfordjournals.jbchem.a131438. [DOI] [PubMed] [Google Scholar]
- Mezgueldi M., Fattoum A., Derancourt J., Kassab R. Mapping of the functional domains in the amino-terminal region of calponin. J Biol Chem. 1992 Aug 5;267(22):15943–15951. [PubMed] [Google Scholar]
- Miller L., Phillips M., Reisler E. Polymerization of G-actin by myosin subfragment 1. J Biol Chem. 1988 Feb 5;263(4):1996–2002. [PubMed] [Google Scholar]
- Nakamura F., Mino T., Yamamoto J., Naka M., Tanaka T. Identification of the regulatory site in smooth muscle calponin that is phosphorylated by protein kinase C. J Biol Chem. 1993 Mar 25;268(9):6194–6201. [PubMed] [Google Scholar]
- Noda S., Ito M., Watanabe S., Takahashi K., Maruyama K. Conformational changes of actin induced by calponin. Biochem Biophys Res Commun. 1992 May 29;185(1):481–487. doi: 10.1016/s0006-291x(05)81010-1. [DOI] [PubMed] [Google Scholar]
- North A. J., Gimona M., Cross R. A., Small J. V. Calponin is localised in both the contractile apparatus and the cytoskeleton of smooth muscle cells. J Cell Sci. 1994 Mar;107(Pt 3):437–444. doi: 10.1242/jcs.107.3.437. [DOI] [PubMed] [Google Scholar]
- Shirinsky V. P., Biryukov K. G., Hettasch J. M., Sellers J. R. Inhibition of the relative movement of actin and myosin by caldesmon and calponin. J Biol Chem. 1992 Aug 5;267(22):15886–15892. [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Stossel T. P., Chaponnier C., Ezzell R. M., Hartwig J. H., Janmey P. A., Kwiatkowski D. J., Lind S. E., Smith D. B., Southwick F. S., Yin H. L. Nonmuscle actin-binding proteins. Annu Rev Cell Biol. 1985;1:353–402. doi: 10.1146/annurev.cb.01.110185.002033. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Hiwada K., Kokubu T. Isolation and characterization of a 34,000-dalton calmodulin- and F-actin-binding protein from chicken gizzard smooth muscle. Biochem Biophys Res Commun. 1986 Nov 26;141(1):20–26. doi: 10.1016/s0006-291x(86)80328-x. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Hiwada K., Kokubu T. Vascular smooth muscle calponin. A novel troponin T-like protein. Hypertension. 1988 Jun;11(6 Pt 2):620–626. doi: 10.1161/01.hyp.11.6.620. [DOI] [PubMed] [Google Scholar]
- Tellam R., Frieden C. Cytochalasin D and platelet gelsolin accelerate actin polymer formation. A model for regulation of the extent of actin polymer formation in vivo. Biochemistry. 1982 Jun 22;21(13):3207–3214. doi: 10.1021/bi00256a027. [DOI] [PubMed] [Google Scholar]
- Wills F. L., McCubbin W. D., Kay C. M. Smooth muscle calponin-caltropin interaction: effect on biological activity and stability of calponin. Biochemistry. 1994 May 10;33(18):5562–5569. doi: 10.1021/bi00184a027. [DOI] [PubMed] [Google Scholar]
- Winder S. J., Walsh M. P. Smooth muscle calponin. Inhibition of actomyosin MgATPase and regulation by phosphorylation. J Biol Chem. 1990 Jun 15;265(17):10148–10155. [PubMed] [Google Scholar]
- YAGI K., MASE R., SAKAKIBARA I., ASAI H. FUNCTION OF HEAVY MEROMYOSIN IN THE ACCELERATION OF ACTIN POLYMERIZATION. J Biol Chem. 1965 Jun;240:2448–2454. [PubMed] [Google Scholar]