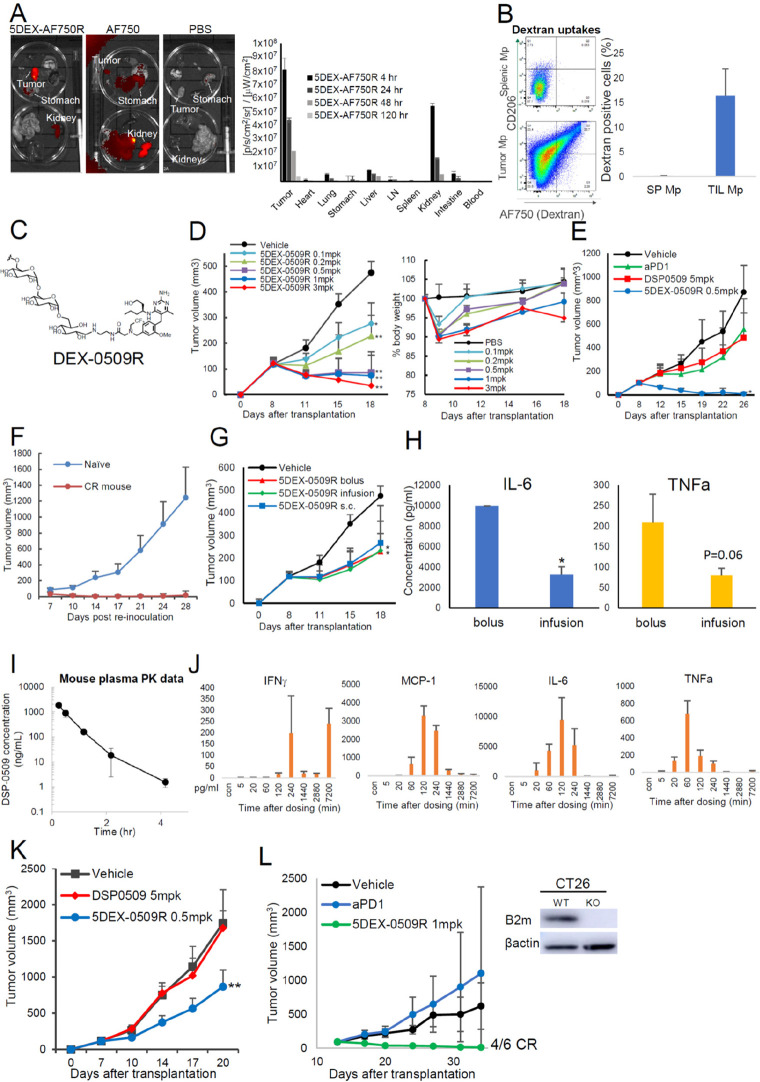
Figure 3.
In vivo profiles and antitumor effect of 5 kDa dextran conjugated with DSP-0509 at the reduced end. (A, B) Tissue and cellular distributions of 5 kDa dextran conjugates. Organ distribution of 5 kDa dextran labeled with AF750 in EMT6 tumor-bearing mice (n = 3). Mice were inoculated with EMT6 tumors and intravenously injected with the dextran-small molecule AF750 complex at 1 mg/kg of AF750 equiv. After 24 h, the blood, heart, thymus, lymph node (LN), spleen, lung, stomach, kidney, and colon were dissected, and their fluorescence intensities were measured. Data are shown as mean ± SD (B) Cellular uptake of 5 kDa dextran conjugates. Mice were inoculated with EMT6 tumors and then intravenously injected with the dextran-AF750 complex 1 mpk of AF750 (n = 3). After 24 h, the tumors and spleens were dissected, dissociated into single -cell suspensions, and then analyzed with flow cytometry. Macrophages were categorized as CD11b+ F4/80+ cells. (C) Chemical structure of DEX-0509R. (D) Dose-dependent antitumor effect of 5DEX-0509R and weight changes in mice injected with 5DEX-0509R. EMT6-bearing mice (n = 6 per group) were intravenously injected once a week for 2 weeks with different doses of 5DEX-0509R (indicated as equivalent dose of DSP-0509). Data are shown as mean ± SD. Statistical differences were evaluated by the parametric Dunnett test vs vehicle (PBS treatment) (*p < 0.05, **p < 0.01). (E) Comparison of the antitumor effect of 5DEX-0509R with that of anti-PD1 and DSP-0509. Colon26-bearing mice (n = 6 per group) were injected with vehicle (PBS, i.v., once a week), anti-PD1 (10 mg/kg, i.p., twice a week), DSP-0509 (5 mg/kg, i.v., once a week), and 5DEX-0509R (0.5 mpk as equivalent dose of DSP-0509, once a week) for 3 weeks. Data are shown as mean ± SD. Statistical differences were evaluated by the parametric Dunnett test vs vehicle (PBS treatment) (*p < 0.01). (F) Reinjection of EMT6 tumor cells into cured mice. Mice showing complete remission of EMT6 tumors by 5DEX-0509R treatment, and naïve mice were injected with EMT6 tumor cells (0.5 × 106 cells/mouse) and monitored. (G, H) Antitumor effect and cytokine profiles of mice injected with 5DEX-0509R via different routes. EMT6-bearing mice (n = 6 per group) were injected once a week for 2 weeks with 5DEX-0509R (0.2 mg/kg as equivalent dose of DSP-0509) by the i.v. bolus, s.c. bolus, and i.v. infusion routes. Data are shown as mean ± SD. Statistical differences were evaluated by the parametric Dunnett test vs vehicle (PBS treatment) (*p < 0.05, **p < 0.01). (H) Plasma cytokine levels of mice injected with 5DEX-0509R via the i.v. bolus and i.v. infusion routes at 2 h after injection. Data are shown as mean ± SD. Statistical differences were evaluated by t-tests. (I, J) Plasma pharmacokinetics and cytokine profiles of 5DEX-0509R. Mouse plasma samples (n = 3) were collected at 10 min, 30 min, 1 h (60 min), 2 h (120 min), 4 h (240 min), 24 h (1440 min), 48 h (2880 min), and 12 h (7200 min) and subjected to (I) pharmacokinetic analysis and (J) cytokine analysis. (K) Antitumor Effect of 5DEX-0509R and DSP-0509. Colon26-bearing nu/nu mice (n = 6 per group) were treated at day 7 with vehicle (PBS, i.v.), DSP-0509 (5 mg/kg, i.v.), or 5DEX-0509R (0.5 mpk as equivalent dose of DSP-0509, i.v.). Data are shown as mean ± SD. Statistical differences were evaluated by the parametric Dunnett test vs vehicle (PBS treatment) (**p < 0.01). (L) Antitumor effect of 5DEX-0509R in the B2M KO CT26 tumor model. B2M knockout of the CT26 cell line was confirmed by Western blotting (left panel). The cells were inoculated, and the tumor-bearing mice (n = 6 per group) were treated with vehicle (PBS, i.v., once a week), DSP-0509 (5 mpk, i.v., once a week), or 5DEX-0509R (1 mpk as equivalent dose of DSP-0509, i.v., once a week) for 3 weeks. Data are shown as mean ± SD.