Abstract
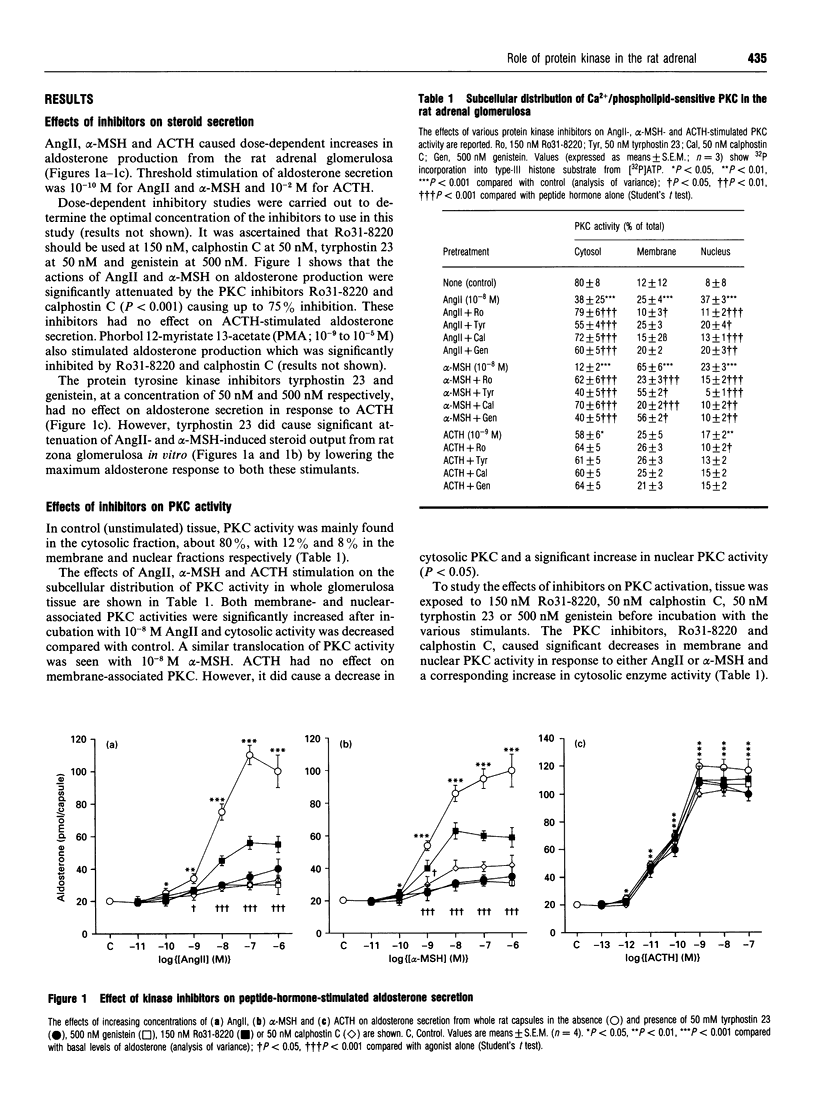
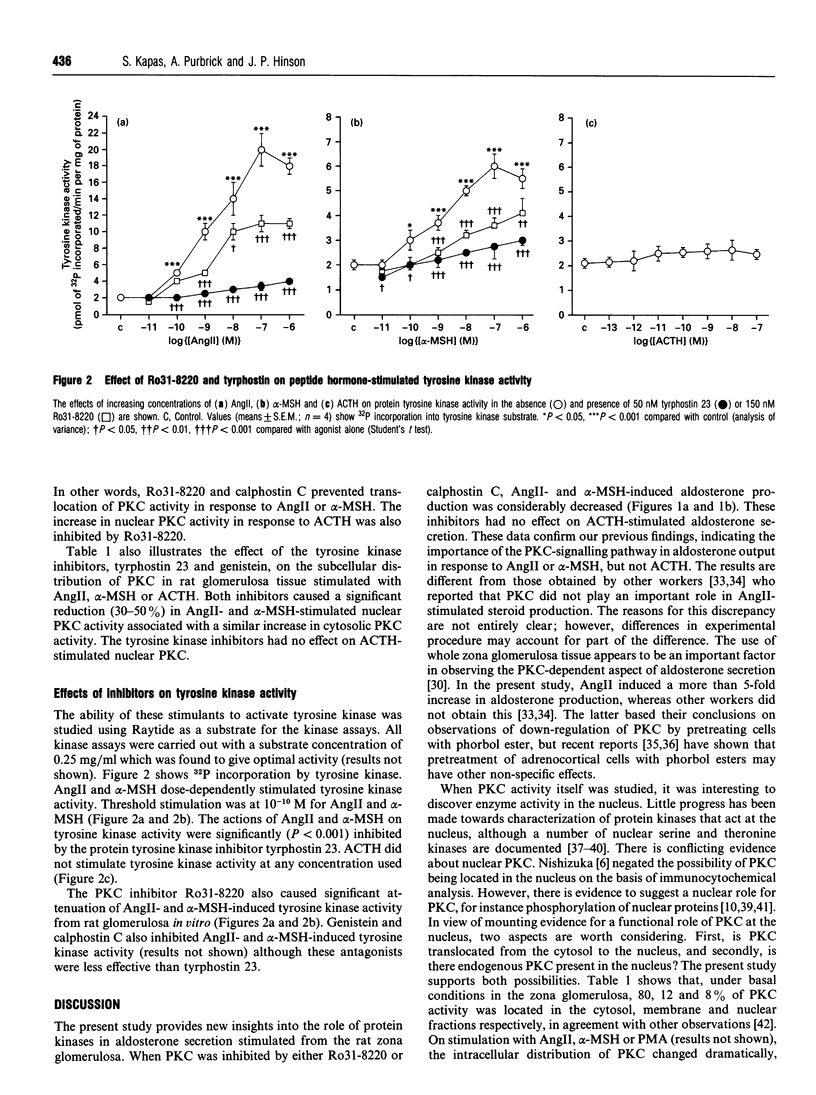
The role of protein kinases in the steroidogenic actions of alpha-melanocyte-stimulating hormone (alpha-MSH), angiotensin II (AngII) and corticotropin (ACTH) in the rat adrenal zona glomerulosa was examined. Ro31-8220, a potent selective inhibitor of protein kinase C (PKC), inhibited both AngII- and alpha-MSH-stimulated aldosterone secretion but had no effect on aldosterone secretion in response to ACTH. The effect of Ro31-8220 on PKC activity was measured in subcellular fractions. Basal PKC activity was higher in cytosol than in membrane or nuclear fractions. Incubation of the zona glomerulosa with either alpha-MSH or AngII resulted in significant increases in PKC activity in the nuclear and cytosolic fractions and decreases in the membrane fraction. These effects were all inhibited by Ro31-8220. ACTH caused a significant increase in nuclear PKC activity only, and this was inhibited by Ro31-8220 without any significant effect on the steroidogenic response to ACTH, suggesting that PKC translocation in response to ACTH may be involved in another aspect of adrenal cellular function. Tyrosine phosphorylation has not previously been considered to be an important component of the response of adrenocortical cells to peptide hormones. Both AngII and alpha-MSH were found to activate tyrosine kinase, but ACTH had no effect, observations that have not been previously reported. Tyrphostin 23, a specific antagonist of tyrosine kinases, inhibited aldosterone secretion in response to AngII and alpha-MSH, but not ACTH. These data confirm the importance of PKC in the adrenocortical response to AngII and alpha-MSH, and, furthermore, indicate that tyrosine kinase may play a critical role in the steroidogenic actions of AngII and alpha-MSH in the rat adrenal zona glomerulosa.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambroz C., Clark A. J., Catt K. J. The mas oncogene enhances angiotensin-induced [Ca2+]i responses in cells with pre-existing angiotensin II receptors. Biochim Biophys Acta. 1991 Dec 3;1133(1):107–111. doi: 10.1016/0167-4889(91)90248-v. [DOI] [PubMed] [Google Scholar]
- Balla T., Baukal A. J., Eng S., Catt K. J. Angiotensin II receptor subtypes and biological responses in the adrenal cortex and medulla. Mol Pharmacol. 1991 Sep;40(3):401–406. [PubMed] [Google Scholar]
- Balla T., Baukal A. J., Guillemette G., Morgan R. O., Catt K. J. Angiotensin-stimulated production of inositol trisphosphate isomers and rapid metabolism through inositol 4-monophosphate in adrenal glomerulosa cells. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9323–9327. doi: 10.1073/pnas.83.24.9323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker S., Marchant W., Ho M. M., Puddefoot J. R., Hinson J. P., Clark A. J., Vinson G. P. A monoclonal antibody to a conserved sequence in the extracellular domain recognizes the angiotensin II AT1 receptor in mammalian target tissues. J Mol Endocrinol. 1993 Oct;11(2):241–245. doi: 10.1677/jme.0.0110241. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Blenis J. Growth-regulated signal transduction by the MAP kinases and RSKs. Cancer Cells. 1991 Nov;3(11):445–449. [PubMed] [Google Scholar]
- Carvallo P., Aguilera G. Protein kinase C mediates the effect of vasopressin in pituitary corticotrophs. Mol Endocrinol. 1989 Dec;3(12):1935–1943. doi: 10.1210/mend-3-12-1935. [DOI] [PubMed] [Google Scholar]
- Cheng H. C., Matsuura I., Wang J. H. In vitro substrate specificity of protein tyrosine kinases. Mol Cell Biochem. 1993 Nov;127-128:103–112. doi: 10.1007/BF01076761. [DOI] [PubMed] [Google Scholar]
- Chiu A. T., Herblin W. F., McCall D. E., Ardecky R. J., Carini D. J., Duncia J. V., Pease L. J., Wong P. C., Wexler R. R., Johnson A. L. Identification of angiotensin II receptor subtypes. Biochem Biophys Res Commun. 1989 Nov 30;165(1):196–203. doi: 10.1016/0006-291x(89)91054-1. [DOI] [PubMed] [Google Scholar]
- Clark A. J., Balla T., Jones M. R., Catt K. J. Stimulation of early gene expression by angiotensin II in bovine adrenal glomerulosa cells: roles of calcium and protein kinase C. Mol Endocrinol. 1992 Nov;6(11):1889–1898. doi: 10.1210/mend.6.11.1336125. [DOI] [PubMed] [Google Scholar]
- Davis P. D., Hill C. H., Keech E., Lawton G., Nixon J. S., Sedgwick A. D., Wadsworth J., Westmacott D., Wilkinson S. E. Potent selective inhibitors of protein kinase C. FEBS Lett. 1989 Dec 18;259(1):61–63. doi: 10.1016/0014-5793(89)81494-2. [DOI] [PubMed] [Google Scholar]
- Enyedi P., Büki B., Muscsi I., Spät A. Polyphosphoinositide metabolism in adrenal glomerulosa cells. Mol Cell Endocrinol. 1985 Jun;41(1):105–112. doi: 10.1016/0303-7207(85)90147-9. [DOI] [PubMed] [Google Scholar]
- Ferrell J. E., Jr, Martin G. S. Platelet tyrosine-specific protein phosphorylation is regulated by thrombin. Mol Cell Biol. 1988 Sep;8(9):3603–3610. doi: 10.1128/mcb.8.9.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell J. E., Jr, Martin G. S. Thrombin stimulates the activities of multiple previously unidentified protein kinases in platelets. J Biol Chem. 1989 Dec 5;264(34):20723–20729. [PubMed] [Google Scholar]
- Force T., Kyriakis J. M., Avruch J., Bonventre J. V. Endothelin, vasopressin, and angiotensin II enhance tyrosine phosphorylation by protein kinase C-dependent and -independent pathways in glomerular mesangial cells. J Biol Chem. 1991 Apr 5;266(10):6650–6656. [PubMed] [Google Scholar]
- Fujita K., Aguilera G., Catt K. J. The role of cyclic AMP in aldosterone production by isolated zona glomerulosa cells. J Biol Chem. 1979 Sep 10;254(17):8567–8574. [PubMed] [Google Scholar]
- Ganguly A., Chiou S., Fineberg N. S., Davis J. S. Greater importance of Ca(2+)-calmodulin in maintenance of ang II- and K(+)-mediated aldosterone secretion: lesser role of protein kinase C. Biochem Biophys Res Commun. 1992 Jan 15;182(1):254–261. doi: 10.1016/s0006-291x(05)80138-x. [DOI] [PubMed] [Google Scholar]
- Glass D. B., Masaracchia R. A., Feramisco J. R., Kemp B. E. Isolation of phosphorylated peptides and proteins on ion exchange papers. Anal Biochem. 1978 Jul 1;87(2):566–575. doi: 10.1016/0003-2697(78)90707-8. [DOI] [PubMed] [Google Scholar]
- Hausdorff W. P., Pitcher J. A., Luttrell D. K., Linder M. E., Kurose H., Parsons S. J., Caron M. G., Lefkowitz R. J. Tyrosine phosphorylation of G protein alpha subunits by pp60c-src. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5720–5724. doi: 10.1073/pnas.89.13.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hug H., Sarre T. F. Protein kinase C isoenzymes: divergence in signal transduction? Biochem J. 1993 Apr 15;291(Pt 2):329–343. doi: 10.1042/bj2910329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. A thousand and one protein kinases. Cell. 1987 Sep 11;50(6):823–829. doi: 10.1016/0092-8674(87)90509-5. [DOI] [PubMed] [Google Scholar]
- Hunyady L., Balla T., Nagy K., Spät A. Control of phosphatidylinositol turnover in adrenal glomerulosa cells. Biochim Biophys Acta. 1982 Nov 12;713(2):352–357. doi: 10.1016/0005-2760(82)90253-3. [DOI] [PubMed] [Google Scholar]
- Imagawa M., Chiu R., Karin M. Transcription factor AP-2 mediates induction by two different signal-transduction pathways: protein kinase C and cAMP. Cell. 1987 Oct 23;51(2):251–260. doi: 10.1016/0092-8674(87)90152-8. [DOI] [PubMed] [Google Scholar]
- Ishii T., Kohno M., Nakamura M., Hinuma Y., Sugamura K. Characterization of interleukin 2-stimulated phosphorylation of 67 and 63 kDa proteins in human T-cells. Biochem J. 1987 Feb 15;242(1):211–219. doi: 10.1042/bj2420211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez E., Marsigliante S., Barker S., Hinson J. P., Vinson G. P. Multiple forms of angiotensin II receptors in rat tissues. J Mol Endocrinol. 1991 Aug;7(1):21–26. doi: 10.1677/jme.0.0070021. [DOI] [PubMed] [Google Scholar]
- Kapas S., Orford C. D., Barker S., Vinson G. P., Hinson J. P. Studies on the intracellular mechanism of action of alpha-melanocyte-stimulating hormone on rat adrenal zona glomerulosa. J Mol Endocrinol. 1992 Aug;9(1):47–54. doi: 10.1677/jme.0.0090047. [DOI] [PubMed] [Google Scholar]
- Kojima I., Kojima K., Rasmussen H. Role of calcium and cAMP in the action of adrenocorticotropin on aldosterone secretion. J Biol Chem. 1985 Apr 10;260(7):4248–4256. [PubMed] [Google Scholar]
- Kolch W., Heidecker G., Kochs G., Hummel R., Vahidi H., Mischak H., Finkenzeller G., Marmé D., Rapp U. R. Protein kinase C alpha activates RAF-1 by direct phosphorylation. Nature. 1993 Jul 15;364(6434):249–252. doi: 10.1038/364249a0. [DOI] [PubMed] [Google Scholar]
- Kyriakis J. M., Force T. L., Rapp U. R., Bonventre J. V., Avruch J. Mitogen regulation of c-Raf-1 protein kinase activity toward mitogen-activated protein kinase-kinase. J Biol Chem. 1993 Jul 25;268(21):16009–16019. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lehoux J. G., Grondin F., Pacuraru J. P., Yachaoui Y. The protein kinase C content is increased in the nuclear fraction of rat adrenal zona glomerulosa following long-term ACTH administration. Mol Cell Endocrinol. 1991 Jun;78(1-2):97–106. doi: 10.1016/0303-7207(91)90190-4. [DOI] [PubMed] [Google Scholar]
- Levitzki A. Tyrphostins--potential antiproliferative agents and novel molecular tools. Biochem Pharmacol. 1990 Sep 1;40(5):913–918. doi: 10.1016/0006-2952(90)90474-y. [DOI] [PubMed] [Google Scholar]
- Macfarlane D. E. Phorbol diester-induced phosphorylation of nuclear matrix proteins in HL60 promyelocytes. Possible role in differentiation studied by cationic detergent gel electrophoresis. J Biol Chem. 1986 May 25;261(15):6947–6953. [PubMed] [Google Scholar]
- Maharajan P., Maharajan V. Growth hormone signal transduction. Experientia. 1993 Nov 15;49(11):980–987. doi: 10.1007/BF02125645. [DOI] [PubMed] [Google Scholar]
- Masmoudi A., Labourdette G., Mersel M., Huang F. L., Huang K. P., Vincendon G., Malviya A. N. Protein kinase C located in rat liver nuclei. Partial purification and biochemical and immunochemical characterization. J Biol Chem. 1989 Jan 15;264(2):1172–1179. [PubMed] [Google Scholar]
- Molloy C. J., Taylor D. S., Weber H. Angiotensin II stimulation of rapid protein tyrosine phosphorylation and protein kinase activation in rat aortic smooth muscle cells. J Biol Chem. 1993 Apr 5;268(10):7338–7345. [PubMed] [Google Scholar]
- Murphy T. J., Alexander R. W., Griendling K. K., Runge M. S., Bernstein K. E. Isolation of a cDNA encoding the vascular type-1 angiotensin II receptor. Nature. 1991 May 16;351(6323):233–236. doi: 10.1038/351233a0. [DOI] [PubMed] [Google Scholar]
- Nakano S., Carvallo P., Rocco S., Aguilera G. Role of protein kinase C on the steroidogenic effect of angiotensin II in the rat adrenal glomerulosa cell. Endocrinology. 1990 Jan;126(1):125–133. doi: 10.1210/endo-126-1-125. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The Albert Lasker Medical Awards. The family of protein kinase C for signal transduction. JAMA. 1989 Oct 6;262(13):1826–1833. [PubMed] [Google Scholar]
- Olashaw N. E., Pledger W. J. Cellular mechanisms regulating proliferation. Adv Second Messenger Phosphoprotein Res. 1988;22:139–173. [PubMed] [Google Scholar]
- Paxton W. G., Marrero M. B., Klein J. D., Delafontaine P., Berk B. C., Bernstein K. E. The angiotensin II AT1 receptor is tyrosine and serine phosphorylated and can serve as a substrate for the src family of tyrosine kinases. Biochem Biophys Res Commun. 1994 Apr 15;200(1):260–267. doi: 10.1006/bbrc.1994.1443. [DOI] [PubMed] [Google Scholar]
- Pelech S. L., Sanghera J. S. Mitogen-activated protein kinases: versatile transducers for cell signaling. Trends Biochem Sci. 1992 Jun;17(6):233–238. doi: 10.1016/s0968-0004(00)80005-5. [DOI] [PubMed] [Google Scholar]
- Persaud S. J., Jones P. M., Sugden D., Howell S. L. Translocation of protein kinase C in rat islets of Langerhans. Effects of a phorbol ester, carbachol and glucose. FEBS Lett. 1989 Mar 13;245(1-2):80–84. doi: 10.1016/0014-5793(89)80196-6. [DOI] [PubMed] [Google Scholar]
- Sasaki K., Yamano Y., Bardhan S., Iwai N., Murray J. J., Hasegawa M., Matsuda Y., Inagami T. Cloning and expression of a complementary DNA encoding a bovine adrenal angiotensin II type-1 receptor. Nature. 1991 May 16;351(6323):230–233. doi: 10.1038/351230a0. [DOI] [PubMed] [Google Scholar]
- Schaller M. D., Bouton A. H., Flynn D. C., Parsons J. T. Identification and characterization of novel substrates for protein tyrosine kinases. Prog Nucleic Acid Res Mol Biol. 1993;44:205–227. doi: 10.1016/s0079-6603(08)60221-4. [DOI] [PubMed] [Google Scholar]
- Shibata H., Kojima I. Involvement of protein kinase C in angiotensin II-mediated release of 12-hydroxyeicosatetraenoic acid in bovine adrenal glomerulosa cells. Endocrinol Jpn. 1991 Dec;38(6):611–617. doi: 10.1507/endocrj1954.38.611. [DOI] [PubMed] [Google Scholar]
- Simonson M. S., Herman W. H. Protein kinase C and protein tyrosine kinase activity contribute to mitogenic signaling by endothelin-1. Cross-talk between G protein-coupled receptors and pp60c-src. J Biol Chem. 1993 May 5;268(13):9347–9357. [PubMed] [Google Scholar]
- Tamm C., Lang U., Vallotton N. B. Effects of atrial natriuretic factor on angiotensin-II-and phorbol ester-stimulated protein kinase-C and prostacyclin production in cultured rat aortic smooth muscle cells. Endocrinology. 1990 Jan;126(1):658–665. doi: 10.1210/endo-126-1-658. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Viard I., Hall S. H., Jaillard C., Berthelon M. C., Saez J. M. Regulation of c-fos, c-jun and jun-B messenger ribonucleic acids by angiotensin-II and corticotropin in ovine and bovine adrenocortical cells. Endocrinology. 1992 Mar;130(3):1193–1200. doi: 10.1210/endo.130.3.1311231. [DOI] [PubMed] [Google Scholar]
- Vinson G. P., Laird S. M., Hinson J. P., Mallick N., Marsigliante S., Teja R. Trypsin stimulation of aldosterone and 18-hydroxycorticosterone production by rat adrenal zona glomerulosa tissue is mediated by activation of protein kinase C. J Mol Endocrinol. 1990 Aug;5(1):85–93. doi: 10.1677/jme.0.0050085. [DOI] [PubMed] [Google Scholar]
- Weber H., Webb M. L., Serafino R., Taylor D. S., Moreland S., Norman J., Molloy C. J. Endothelin-1 and angiotensin-II stimulate delayed mitogenesis in cultured rat aortic smooth muscle cells: evidence for common signaling mechanisms. Mol Endocrinol. 1994 Feb;8(2):148–158. doi: 10.1210/mend.8.2.8170471. [DOI] [PubMed] [Google Scholar]
- Whitebread S., Mele M., Kamber B., de Gasparo M. Preliminary biochemical characterization of two angiotensin II receptor subtypes. Biochem Biophys Res Commun. 1989 Aug 30;163(1):284–291. doi: 10.1016/0006-291x(89)92133-5. [DOI] [PubMed] [Google Scholar]
- Wilkinson S. E., Hallam T. J. Protein kinase C: is its pivotal role in cellular activation over-stated? Trends Pharmacol Sci. 1994 Feb;15(2):53–57. doi: 10.1016/0165-6147(94)90110-4. [DOI] [PubMed] [Google Scholar]
- Wilkinson S. E., Parker P. J., Nixon J. S. Isoenzyme specificity of bisindolylmaleimides, selective inhibitors of protein kinase C. Biochem J. 1993 Sep 1;294(Pt 2):335–337. doi: 10.1042/bj2940335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L. T. Signal transduction by the platelet-derived growth factor receptor. Science. 1989 Mar 24;243(4898):1564–1570. doi: 10.1126/science.2538922. [DOI] [PubMed] [Google Scholar]
- Yang G. A., Koistinaho J., Iadarola M., Shenhua-Zhu, Hervonen A. Administration of adrenocorticotropic hormone (ACTH) enhances Fos expression in the rat adrenal cortex. Regul Pept. 1990 Aug 21;30(1):21–31. doi: 10.1016/0167-0115(90)90132-g. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Ullrich A. Growth factor receptor tyrosine kinases. Annu Rev Biochem. 1988;57:443–478. doi: 10.1146/annurev.bi.57.070188.002303. [DOI] [PubMed] [Google Scholar]


