Figure 1.
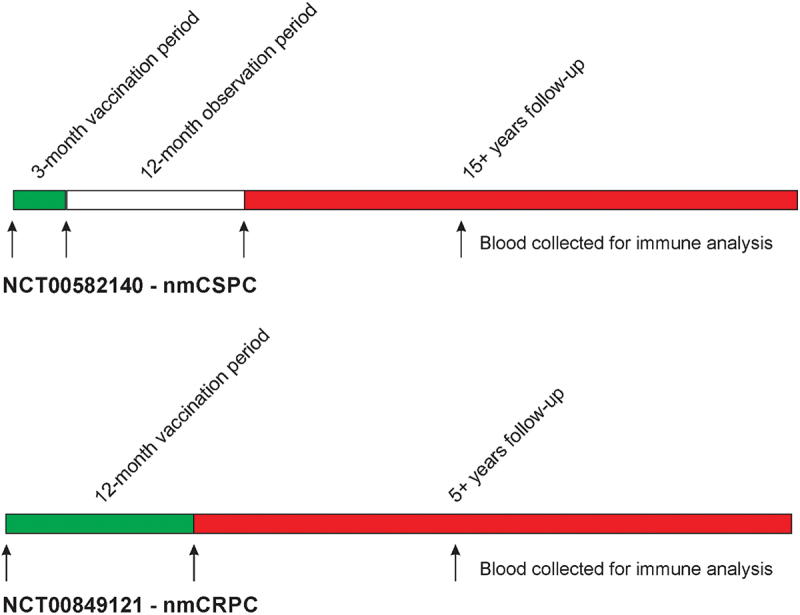
Schemas. Shown are the original trial schemas and long-term follow-up. In the nmCSPC trial, 6 vaccinations were delivered every 2 weeks over a 3-month vaccination course, and patients were then monitored for immune responses over an additional 12 months.12 In the nmCRPC trial, 6 vaccinations were delivered every 2 weeks over a 3-month vaccination course, and then, patients received either fixed booster immunizations every 3 months for 1 y total, or received vaccinations continuing every 2 weeks until evidence of immune response, with the schedule of immunization effectively being determined by immune monitoring.19 Cryopreserved blood samples for immune analysis were available pre-treatment, at the end of the treatment period, and at later time points during long-term follow-up.
