Abstract
Equilibrium and kinetic fluorescence methods have been used to characterize the interactions between K+ and the Ca(2+)-ATPase of skeletal-muscle sarcoplasmic reticulum. K+ shifts the E2-E1 equilibrium of the ATPase towards E1 and increases the rate of Ca2+ binding to the ATPase, as detected by changes in tryptophan fluorescence intensity, suggesting that K+ increases the rate of the E2-E1 transition. The data are consistent with binding of K+ at the inner Ca(2+)-binding site on the ATPase in competition with H+ and Mg2+, with a higher affinity in the E1 than in the E2 conformation. K+ has no effect on the affinity for Mg2+, as detected by changes in tryptophan fluorescence intensity; since it has been proposed that the changes in tryptophan fluorescence follow from binding to Mg2+ at the outer Ca(2+)-binding site, this suggests that K+ is unable to bind at the outer Ca(2+)-binding site. K+ increases the rate of dissociation of Ca2+ from the Ca(2+)-bound ATPase and reduces the effect of Mg2+ on the fluorescence intensity of the ATPase labelled with 4-(bromomethyl)-6,7-dimethoxycoumarin. It is suggested that these effects of K+ are the result of binding at a 'gating' site on the ATPase, in competition with binding of H+. Binding of K+ at the inner Ca(2+)-binding site and at the gating site account for the observed effects of K+ on the affinity of the ATPase for Ca2+.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cha S. A simple method for derivation of rate equations for enzyme-catalyzed reactions under the rapid equilibrium assumption or combined assumptions of equilibrium and steady state. J Biol Chem. 1968 Feb 25;243(4):820–825. [PubMed] [Google Scholar]
- Chaloub R. M., de Meis L. Effect of K+ on phosphorylation of the sarcoplasmic reticulum ATPase by either Pi or ATP. J Biol Chem. 1980 Jul 10;255(13):6168–6172. [PubMed] [Google Scholar]
- Champeil P., Gingold M. P., Guillain F., Inesi G. Effect of magnesium on the calcium-dependent transient kinetics of sarcoplasmic reticulum ATPase, studied by stopped flow fluorescence and phosphorylation. J Biol Chem. 1983 Apr 10;258(7):4453–4458. [PubMed] [Google Scholar]
- Delville A., Grandjean J., Laszlo P., Gerday C., Grabarek Z., Drabikowski W. Sodium-23 nuclear magnetic resonance as an indicator of sodium binding to troponin C and tryptic fragments, in relation to calcium content and attendant conformational changes. Eur J Biochem. 1980 Apr;105(2):289–295. doi: 10.1111/j.1432-1033.1980.tb04500.x. [DOI] [PubMed] [Google Scholar]
- Duggan P. F. Caclium uptake and associated adenosine triphosphatase activity in fragmented sarcoplasmic reticulum. Requirement for potassium ions. J Biol Chem. 1977 Mar 10;252(5):1620–1627. [PubMed] [Google Scholar]
- Dupont Y., Guillain F., Lacapere J. J. Fluorimetric detection and significance of conformational changes in Ca2+-ATPase. Methods Enzymol. 1988;157:206–219. doi: 10.1016/0076-6879(88)57076-3. [DOI] [PubMed] [Google Scholar]
- Dupont Y., Leigh J. B. Transient kinetics of sarcoplasmic reticulum CA2+ + Mg2+ ATPase studied by fluorescence. Nature. 1978 Jun 1;273(5661):396–398. doi: 10.1038/273396a0. [DOI] [PubMed] [Google Scholar]
- Dupont Y. Low-temperature studies of the sarcoplasmic reticulum calcium pump. Mechanisms of calcium binding. Biochim Biophys Acta. 1982 May 21;688(1):75–87. doi: 10.1016/0005-2736(82)90580-6. [DOI] [PubMed] [Google Scholar]
- Fernandez-Belda F., Kurzmack M., Inesi G. A comparative study of calcium transients by isotopic tracer, metallochromic indicator, and intrinsic fluorescence in sarcoplasmic reticulum ATPase. J Biol Chem. 1984 Aug 10;259(15):9687–9698. [PubMed] [Google Scholar]
- Forge V., Mintz E., Guillain F. Ca2+ binding to sarcoplasmic reticulum ATPase revisited. I. Mechanism of affinity and cooperativity modulation by H+ and Mg2+. J Biol Chem. 1993 May 25;268(15):10953–10960. [PubMed] [Google Scholar]
- Froud R. J., Lee A. G. Conformational transitions in the Ca2+ + Mg2+-activated ATPase and the binding of Ca2+ ions. Biochem J. 1986 Jul 1;237(1):197–206. doi: 10.1042/bj2370197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godt R. E. Calcium-activated tension of skinned muscle fibers of the frog. Dependence on magnesium adenosine triphosphate concentration. J Gen Physiol. 1974 Jun;63(6):722–739. doi: 10.1085/jgp.63.6.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M. A., Tomlins B., Montgomery R. A., Williams A. J. Structural aspects of the sarcoplasmic reticulum K+ channel revealed by gallamine block. Biophys J. 1988 Aug;54(2):233–239. doi: 10.1016/S0006-3495(88)82952-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillain F., Champeil P., Lacapère J. J., Gingold M. P. Stopped flow and rapid quenching measurement of the transient steps induced by calcium binding to sarcoplasmic reticulum adenosine triphosphatase. Competition with Ca2+-independent phosphorylation. J Biol Chem. 1981 Jun 25;256(12):6140–6147. [PubMed] [Google Scholar]
- Guillain F., Gingold M. P., Büschlen S., Champeil P. A direct fluorescence study of the transient steps induced by calcium binding to sarcoplasmic reticulum ATPase. J Biol Chem. 1980 Mar 10;255(5):2072–2076. [PubMed] [Google Scholar]
- Guillain F., Gingold M. P., Champeil P. Direct fluorescence measurements of Mg2+ binding to sarcoplasmic reticulum ATPase. J Biol Chem. 1982 Jul 10;257(13):7366–7371. [PubMed] [Google Scholar]
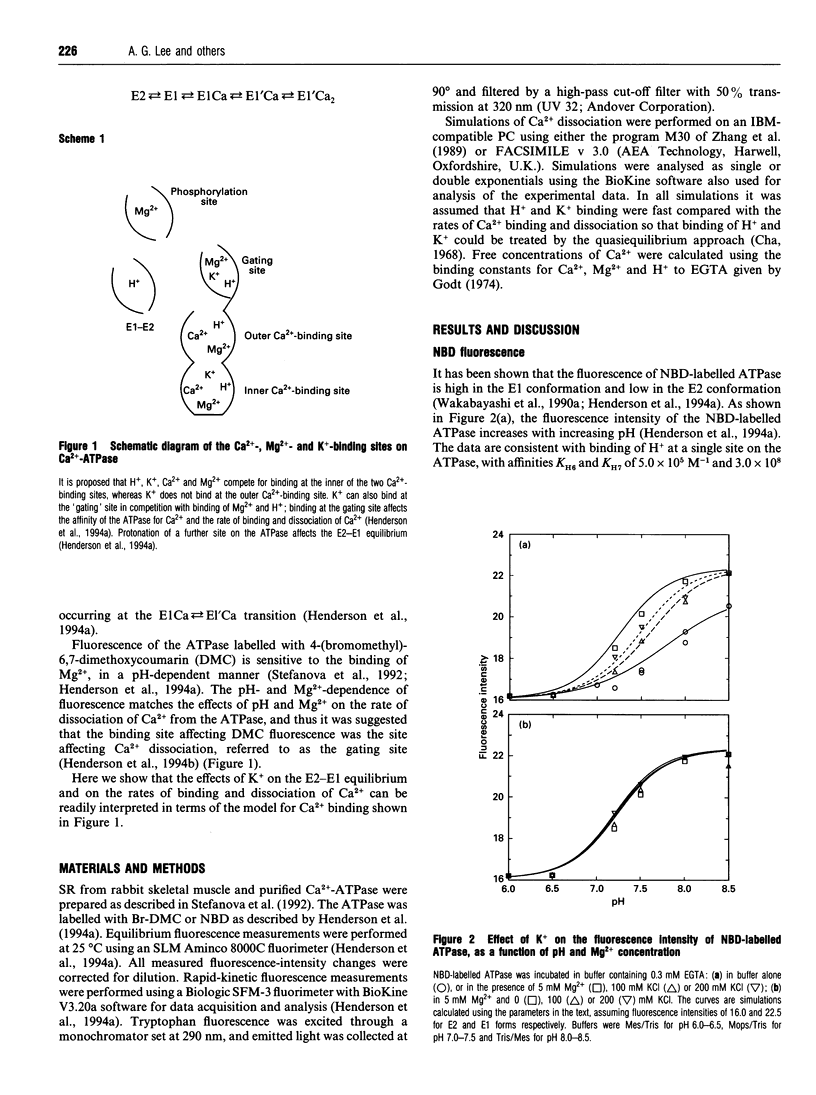
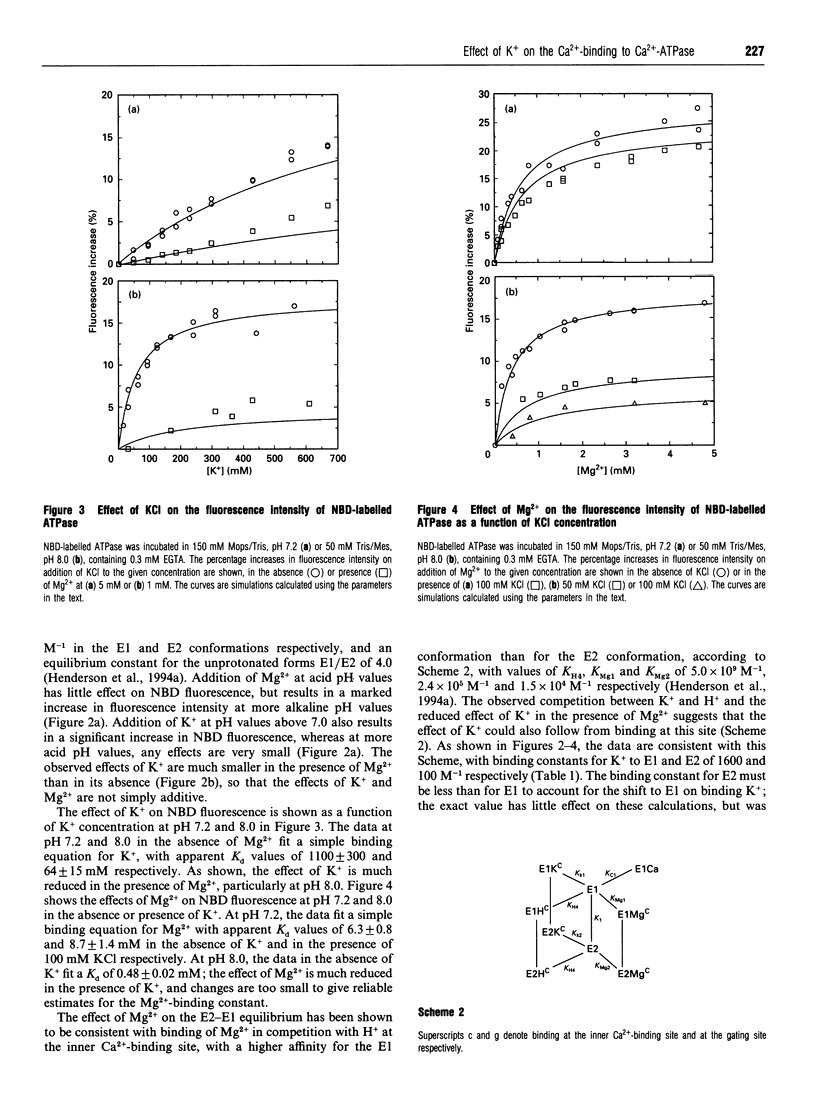
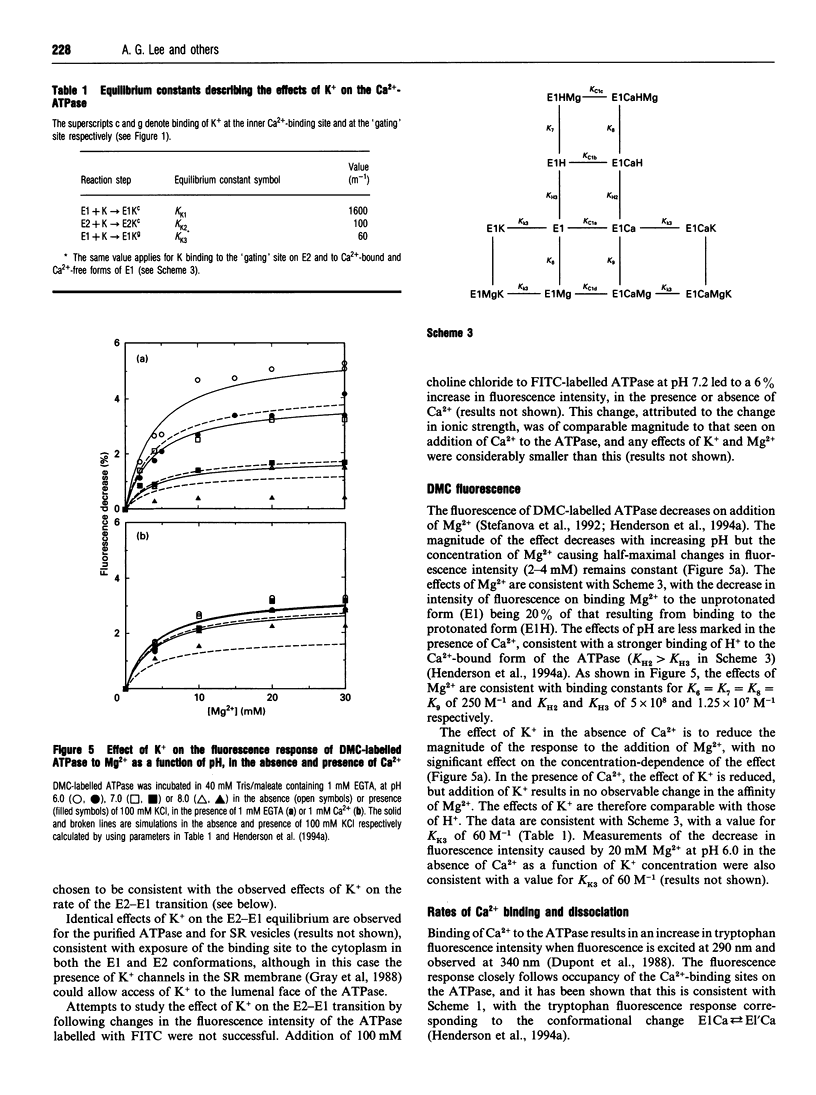
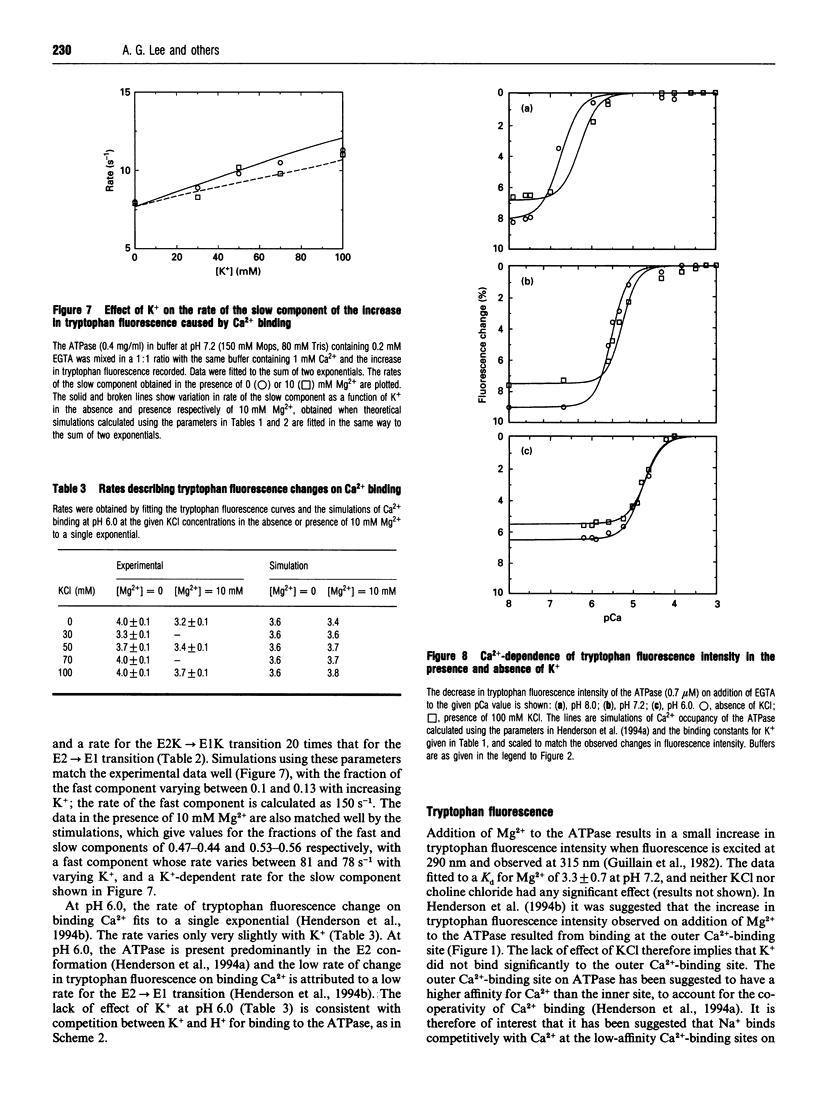
- Henderson I. M., Khan Y. M., East J. M., Lee A. G. Binding of Ca2+ to the (Ca(2+)-Mg2+)-ATPase of sarcoplasmic reticulum: equilibrium studies. Biochem J. 1994 Feb 1;297(Pt 3):615–624. doi: 10.1042/bj2970615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson I. M., Starling A. P., Wictome M., East J. M., Lee A. G. Binding of Ca2+ to the (Ca(2+)-Mg2+)-ATPase of sarcoplasmic reticulum: kinetic studies. Biochem J. 1994 Feb 1;297(Pt 3):625–636. doi: 10.1042/bj2970625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inesi G. Sequential mechanism of calcium binding and translocation in sarcoplasmic reticulum adenosine triphosphatase. J Biol Chem. 1987 Dec 5;262(34):16338–16342. [PubMed] [Google Scholar]
- Medda P., Fassold E., Hasselbach W. The effect of monovalent and divalent cations on the ATP-dependent Ca2+-binding and phosphorylation during the reaction cycle of the sarcoplasmic reticulum Ca2+-transport ATPase. Eur J Biochem. 1987 Jun 1;165(2):251–259. doi: 10.1111/j.1432-1033.1987.tb11435.x. [DOI] [PubMed] [Google Scholar]
- Moutin M. J., Dupont Y. Interaction of potassium and magnesium with the high affinity calcium-binding sites of the sarcoplasmic reticulum calcium-ATPase. J Biol Chem. 1991 Mar 25;266(9):5580–5586. [PubMed] [Google Scholar]
- Orlowski S., Champeil P. Kinetics of calcium dissociation from its high-affinity transport sites on sarcoplasmic reticulum ATPase. Biochemistry. 1991 Jan 15;30(2):352–361. doi: 10.1021/bi00216a007. [DOI] [PubMed] [Google Scholar]
- Pick U., Karlish S. J. Regulation of the conformation transition in the Ca-ATPase from sarcoplasmic reticulum by pH, temperature, and calcium ions. J Biol Chem. 1982 Jun 10;257(11):6120–6126. [PubMed] [Google Scholar]
- Pick U. The interaction of vanadate ions with the Ca-ATPase from sarcoplasmic reticulum. J Biol Chem. 1982 Jun 10;257(11):6111–6119. [PubMed] [Google Scholar]
- Ribeiro J. M., Vianna A. L. Allosteric modification by K+ of the (Ca2+ + Mg2+)-dependent ATPase of sarcoplasmic reticulum. Interaction with Mg2+. J Biol Chem. 1978 May 10;253(9):3153–3157. [PubMed] [Google Scholar]
- SRETER F. A. CELL WATER, SODIUM, AND POTASSIUM IN STIMULATED RED AND WHITE MAMMALIAN MUSCLES. Am J Physiol. 1963 Dec;205:1295–1298. doi: 10.1152/ajplegacy.1963.205.6.1295. [DOI] [PubMed] [Google Scholar]
- Scofano H., Barrabin H., Inesi G., Cohen J. A. Stoichiometric and electrostatic characterization of calcium binding to native and lipid-substituted adenosinetriphosphatase of sarcoplasmic reticulum. Biochim Biophys Acta. 1985 Sep 25;819(1):93–104. doi: 10.1016/0005-2736(85)90199-3. [DOI] [PubMed] [Google Scholar]
- Shigekawa M., Dougherty J. P., Katz A. M. Reaction mechanism of Ca2+-dependent ATP hydrolysis by skeletal muscle sarcoplasmic reticulum in the absence of added alkali metal salts. I. Characterization of steady state ATP hydrolysis and comparison with that in the presence of KCl. J Biol Chem. 1978 Mar 10;253(5):1442–1450. [PubMed] [Google Scholar]
- Shigekawa M., Pearl L. J. Activation of calcium transport in skeletal muscle sarcoplasmic reticulum by monovalent cations. J Biol Chem. 1976 Nov 25;251(22):6947–6952. [PubMed] [Google Scholar]
- Stefanova H. I., East J. M., Gore M. G., Lee A. G. Labeling the (Ca(2+)-Mg2+)-ATPase of sarcoplasmic reticulum with 4-(bromomethyl)-6,7-dimethoxycoumarin: detection of conformational changes. Biochemistry. 1992 Jul 7;31(26):6023–6031. doi: 10.1021/bi00141a010. [DOI] [PubMed] [Google Scholar]
- Timonin I. M., Dvoryantsev S. N., Petrov V. V., Ruuge E. K., Levitsky D. O. Interaction of alkaline metal ions with Ca(2+)-binding sites of Ca(2+)-ATPase of sarcoplasmic reticulum: 23Na-NMR studies. Biochim Biophys Acta. 1991 Jul 1;1066(1):43–53. doi: 10.1016/0005-2736(91)90248-7. [DOI] [PubMed] [Google Scholar]
- Verjovski-Almeida S., Silva J. L. Different degrees of cooperativity of the Ca2+-induced changes in fluorescence intensity of solubilized sarcoplasmic reticulum ATPase. J Biol Chem. 1981 Mar 25;256(6):2940–2944. [PubMed] [Google Scholar]
- Wakabayashi S., Imagawa T., Shigekawa M. Does fluorescence of 4-nitrobenzo-2-oxa-1,3-diazole incorporated into sarcoplasmic reticulum ATPase monitor putative E1-E2 conformational transition? J Biochem. 1990 Apr;107(4):563–571. doi: 10.1093/oxfordjournals.jbchem.a123087. [DOI] [PubMed] [Google Scholar]
- Wakabayashi S., Ogurusu T., Shigekawa M. Participation of H+ in the Ca2(+)-induced conformational transition of 4-nitro-2,1,3-benzoxadiazole-labeled sarcoplasmic reticulum ATPase. Biochemistry. 1990 Nov 27;29(47):10613–10620. doi: 10.1021/bi00499a006. [DOI] [PubMed] [Google Scholar]
- Wang T., Grassi de Gende A. O., Tsai L. I., Schwartz A. Influence of monovalent cations on the Ca2+-ATPase of sarcoplasmic reticulum isolated from rabbit skeletal and dog cardiac muscles. An interpretation of transient-state kinetic data. Biochim Biophys Acta. 1981 Oct 12;637(3):523–529. doi: 10.1016/0005-2728(81)90059-1. [DOI] [PubMed] [Google Scholar]
- Wictome M., Michelangeli F., Lee A. G., East J. M. The inhibitors thapsigargin and 2,5-di(tert-butyl)-1,4-benzohydroquinone favour the E2 form of the Ca2+,Mg(2+)-ATPase. FEBS Lett. 1992 Jun 15;304(2-3):109–113. doi: 10.1016/0014-5793(92)80599-c. [DOI] [PubMed] [Google Scholar]
- Zhang X. Z., Strand A., White H. D. A general pre-steady-state solution to complex kinetic mechanisms. Anal Biochem. 1989 Feb 1;176(2):427–431. doi: 10.1016/0003-2697(89)90336-9. [DOI] [PubMed] [Google Scholar]


