Abstract
The early identification of individuals with radiologically isolated syndrome (RIS) who are at an elevated risk of progressing to multiple sclerosis (MS) is essential for making informed treatment decisions. This study aimed to evaluate the predictive potential of multifocal Visual Evoked Potentials (mfVEP) measures in individuals with RIS with respect to their conversion to MS. A prospective observational cohort study was conducted, involving 21 individuals with RIS recruited from a MS center. Baseline assessments, including mfVEP, magnetic resonance imaging (MRI), and clinical examinations, were performed, and participants were longitudinally followed for up to 24 months. The primary outcome measures were the conversion to MS. Over a clinical follow-up period of 24 months, five individuals (5/21) with RIS progressed to MS. MfVEP amplitude responses (interocular and monocular probability analysis) demonstrated abnormal cluster visual field defects in 47.6% of RIS eyes at baseline, whereas multifocal VEP latency analysis showed significant delays in 38.4%. A reduction in interocular amplitude [OR = 0.036, (95% CI 0.003–0.503); P = 0.014], monocular amplitude [OR = 0.083, (95% CI 0.007–0.982); P = 0.048], and a prolonged interocular latency [OR = 0.095, (95% CI 0.009–0.972); P = 0.047] were associated with a higher relative risk of clinical conversion at the 2-year follow-up. Multifocal VEP may serve as a novel and independent risk factor for predicting the conversion to MS in individuals with Radiologically Isolated Syndrome.
Keywords: Radiologically isolated syndrome, Visual afferent pathway, Multifocal visual-evoked potentials
Subject terms: Visual system, Multiple sclerosis
Introduction
Patients with Radiologically Isolated Syndrome (RIS) face an increased risk of transitioning to Multiple Sclerosis (MS). Limited research has explored the role of visual evoked potentials (VEP) in RIS patients, particularly in assessing the risk of conversion to Clinically Isolated Syndrome (CIS) and MS.
The afferent visual pathway serves as a valuable model for studying RIS due to several reasons: (1) it is a well-defined neural pathway with few neurons and long tracts, facilitating the quantification of myelin, axonal, and neuronal defects; (2) it exhibits retinotopic organization across all tracts, enabling precise mapping of damage from the retina to the occipital cortex and aiding in analyzing structure–function correlations crucial for assessing clinical relevance; (3) visual information travels from the retina to the primary visual cortex through a single synapse in the lateral geniculate body, unlike other pathways such as the primary motor pathway, where information is modulated not only by the thalamus but also by the basal ganglia, cerebellum, and spinal cord; (4) the primary visual cortex offers a degree of neuroplasticity within the visual system; and (5) nerve fibers in the retina lack myelin, making it an ideal model for evaluating the interaction between inflammation and axonal damage.
In the 2017 revision of the McDonald Criteria, conventional VEP were not incorporated into the diagnostic criteria for MS, as their inclusion appeared to reduce the specificity of the diagnostic algorithm. However, numerous studies have demonstrated the utility of monitoring the afferent visual pathway through VEP in evaluating the progression of demyelinating pathology1–3. While VEP currently play a limited role in clinical decision-making, they provide valuable insights into the pathophysiological changes in the afferent visual system during the early stages of demyelinating disorders.
VEPs are essential for assessing the extent of demyelination along the afferent visual pathway and can be used to predict the degree of recovery following optic neuritis3. VEPs can detect the incapacitating effects of clinical and subclinical demyelination, serving as a tool to identify patients with RIS at a higher risk of clinical conversion4. The delay in latencies of VEP signals is a reliable indicator of demyelination processes in the visual pathway, and its severity correlates with the extent of elongation in bioelectrical signal latencies5–7. However, changes in latencies often do not correlate with any noticeable clinical visual dysfunction in these early stages. In lesions of the afferent visual pathway, concurrent phenomena of demyelination and axonal degeneration are observed, making it challenging to distinguish the effect of both processes in VEP signals. Normalization of latency values over time indicates the impact of active remyelination processes in the visual system. Amplitudes can also recover in these initial stages upon unblocking conduction, provided that axons are not damaged8. Experimental evidence suggests a significant proportion of ganglion cell axons can be critically damaged in these initial episodes of subclinical acute inflammation of the visual pathway9,10. These axons undergo slow and progressive degeneration, both retrograde and anterograde, and clinical manifestation of this axonal damage may be delayed11.
The visual pathway can be functionally evaluated with the relatively new multifocal VEP. The multifocal VEP offer a functional evaluation of the visual pathway, providing a new perspective. Multifocal VEP have demonstrated greater diagnostic sensitivity for detecting defects in the visual field compared to conventional VEP12. However, the test requires more time to administer, with a steeper learning curve4,13. Currently, only a limited number of hospitals have access to multifocal VEP equipment, making it relatively unknown among ophthalmologists and neurologists. In our clinical experience, the applications of this technology include: (1) differential diagnosis of ischemic, compressive, and inflammatory optic neuropathies; (2) diagnosis and monitoring of afferent visual pathway involvement in Multiple Sclerosis; (3) diagnostic study of idiopathic visual pathway pathology, in conjunction with multifocal electroretinography; and (4) diagnosis of glaucomatous optic neuropathy.
Enhancing our understanding of the damage to the anterior visual pathway and the brain, as revealed by multifocal VEP in these subjects, would be invaluable. This information may serve as potential predictors of disease progression in RIS Syndrome.
Results
Demographics and clinical characteristics
The primary demographic, clinical, and neuroradiological data of the patients with RIS selected for our study are summarized in Table 1. A total of 21 patients were recruited for this study, comprising 3 (14.3%) males and 18 (85.7%) females, with a median and interquartile range (IQR) age of 42.7 (12.3) years. The main reasons for the initial referral to the neurologist were headaches (47.6%, 10/21), vertigo (14.3%, 3/21), fibromyalgia (9.5%, 2/21), and hearing loss (9.5%, 2/21). Less frequent reasons included anosmia (4.8%, 1/21), psychiatric syndromes (4.8%, 1/21), and post-traumatic head injury (4.8%, 1/21). Of the patients, 14.29% (3/21) had a history of autoimmune diseases: two had hypothyroidism (9.5%), and one was diagnosed with inflammatory bowel disease (4.8%). Additionally, two patients (9.5%) were frequent moderate smokers. Baseline brain and/or spinal cord MRI revealed various abnormalities. Specifically, more than nine lesions in T2 mode were observed in 85.7% (18/21) of patients, more than one lesion in T1 mode in 76.2% (16/21), more than one gadolinium-enhancing lesion in 28.6% (6/21), more than one spinal lesion in 14.3% (3/21), and more than one infratentorial lesion in 9.5% (2/21) of cases. Cerebrospinal fluid analysis detected the presence of oligoclonal bands in 44.4% (8/18) of patients and elevated levels of immunoglobulin G in 27.8% (5/21).
Table 1.
Demographic, clinical, and neuroradiological data of RIS patients registered at the study baseline.
| Categories | Subcategories | Count (%) |
|---|---|---|
| Sex | Male | 3 (14.3) |
| Female | 18 (85.7) | |
| Age (years) | Median (IQR) | 42.7 (12.3) |
| > 37 years | 14 (66.7) | |
| ≤ 37 years | 7 (33.3) | |
| Reason for consultation | Headaches | 10 (47.6) |
| Fibromyalgia | 2 (9.5) | |
| Anxiety syndrome | 1 (4.8) | |
| Vertigo | 3 (14.3) | |
| Hearing loss | 2 (9.5) | |
| Head trauma | 1 (4.8) | |
| Anosmia | 1 (4.8) | |
| Psychotic Episode | 1 (4.8) | |
| Presence of autoimmune diseases | Yes | 3 (14.3)) |
| Smoking habit | Yes | 2 (9.5) |
| MRI findings | > 9 lesions in T2 mode | 18 (85.7) |
| > 1 gadolinium-enhancing lesion | 6 (28.6) | |
| > 1 spinal cord lesion | 3 (14.3) | |
| > 1 infratentorial lesion | 2 (9.5%) | |
| > 1 lesion in T1 mode | 16 (76.2) |
Multifocal visual evoked potentials responses
The interocular amplitude analysis (comparing the study eye with its contralateral eye) showed a significant decrease in the amplitudes of multifocal VEP in 28.6% (6/21) of RIS patients compared to the normative control baseline at the first study visit (Fisher's exact test, P = 0.01) (Table 2). The monocular amplitude analysis (comparing the study eye with the normative signal base) demonstrated a significant decrease in multifocal VEP amplitudes in 38.1% (8/21) of patients at the first study visit (Fisher's exact test, P = 0.001). The combined interocular and monocular amplitude analysis showed a significant decrease in 47.6% (6/21) of patients with RIS compared to the normative control baseline at the first study visit (Fisher's exact test, P = 0.001).
Table 2.
Patients with defects identified in the analysis of amplitudes and latencies of Multifocal VEP responses conducted at the first study visit.
| Multifocal VEP analysis | Amplitude (n = 21) | Latency (n + 21) |
|---|---|---|
| Interocular probability plot, no. (%) | 6 (28.6) | 5 (23.8) |
| Monocular probability plot, no. (%) | 8 (38.1) | 5 (23.8) |
| Interocular and monocular probability plots, no. (%) | 10 (47.6) | 8 (38.1) |
The interocular latency analysis (comparing one eye with its contralateral) revealed a significant delay in the latencies of multifocal VEP signals in 23.8% (5/21) of patients with RIS at the first study visit (Fisher's exact test, P = 0.02). The monocular latency analysis (comparing the study eye with the normative signal base) showed a significant delay in latencies in 23.8% (5/21) of patients at the first study visit (Fisher's exact test, P = 0.01). The combined Interocular and monocular latency analysis indicated a significant delay in 38.1% (8/21) of patients with RIS at the first study visit (Fisher's exact test, P = 0.001) (Fig. 1). The combined analysis of interocular and monocular amplitudes and latencies demonstrated significant changes in 61.9% (13/21) of patients with RIS compared to the normative control baseline at the first study visit (Fisher's exact test, P = 0.001). No significant associations were found between interocular and monocular amplitude and latency analysis of multifocal VEP and clinical variables (age, sex, cerebrospinal fluid), MRI variables (T2 lesions, gadolinium-enhancing lesions, spinal lesions, infratentorial lesions, T1 lesions), (Fisher's exact test; P > 0.055) (Table 3).
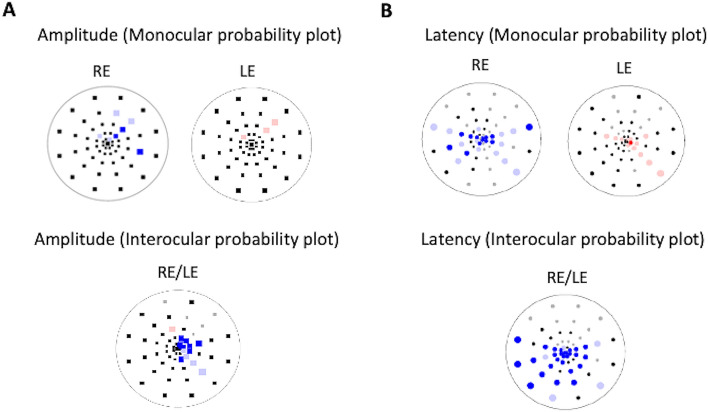
Figure 1.
mfVEP recordings (array of 60 signals from the best channel) taken from a RIS study subject. In the multifocal VEP (interocular amplitude, monocular amplitude and latency analysis probability plots), abnormal clusters are shown in both eyes (RE: right eye; LE: left eye).
Table 3.
Associations of defects in the Multifocal VEP's amplitudes and latencies with clinical, neuroradiological, and ophthalmological variables.
| Variables | Interocular amplitudes | Monocular amplitudes | Interocular latencies | Monocular latencies |
|---|---|---|---|---|
| ≤ 37 years | 0.354 | 1.000 | 0.025 | 0.280 |
| Pathological CSF (OCB + IgG) | 1.000 | 0.131 | 1.000 | 0.294 |
| Nuclear magnetic imaging | ||||
| > 9 lesions in T2 mode | 0.184 | 0.257 | 1.000 | 1.000 |
| > 1 Lesion gadolinium+ | 1.000 | 0.631 | 0.598 | 1.000 |
| > 1 medullary lesion | 0.184 | 0.042 | 0.128 | 0.128 |
| > 1 infratentorial lesion | 1.000 | 1.000 | 1.000 | 1.000 |
| > 1 lesion in T1 mode | 1.000 | 0.606 | 1.000 | 0.278 |
Fisher's exact test was employed for statistical analysis.
CSF cerebrospinal fluid, OCB oligoclonal bands, IgG immunoglobulin G, MRI Magnetic Resonance Imaging, OCT Optical Coherence Tomography, RNFL retinal nerve fiber layer.
At the 12-month follow-up, interocular and monocular amplitude and latency analysis of mfVEPs showed no significant differences compared to baseline results (McNemar's test, P = 1.000). Similarly, no significant differences were observed in the interocular mean latency (Wilcoxon's test, P = 0.73) and monocular mean latency (Wilcoxon's test, P = 0.69) of aggregated signals from the 60 sectors of mfVEPs compared to baseline results. At the end of the study, 24% (5/21) of patients with Isolated Radiological Syndrome clinically converted. Within the first year of follow-up, three patients converted to MS, and in the second year, two patients converted, one to CIS and the other to MS. Patients with RIS who clinically converted at 24 months of follow-up had a median (IQR) age of 32 (13.3) years, while those who did not convert had a median age of 44.7 (8.8) years, with the difference being statistically significant (U-Mann–Whitney test, P = 0.01).
We compared baseline MRI results between patients with RIS who did not convert and those who converted to more advanced forms of demyelinating disease at 24 months of follow-up (Table 4). No statistically significant differences were found in any of the analyzed MRI variables (Fisher's test; > 9 lesions in T2, P = 1.000; gadolinium-enhancing lesions, P = 0.115; spinal lesions, P = 0.128; infratentorial lesions, P = 1.000; and T1 lesions, P = 0.553). Similarly, no significant differences were observed between patients with RIS who converted and those who did not convert clinically at 24 months of follow-up based on the presence of oligoclonal bands and/or elevated IgG levels in the baseline cerebrospinal fluid examination (Fisher's test, P = 1.000). No significant differences were observed in patients with RIS who clinically converted and those who did not convert at 24 months of follow-up in the presence of oligoclonal bands and/or a high immunoglobulin G index in the baseline cerebrospinal fluid examination (Fisher's exact test, P = 0.128).
Table 4.
Differences in baseline results of the MRI in RIS patients who clinically converted and those who did not at 24 months of follow-up.
| Nuclear magnetic imaging | Clinical conversion (N = 5) | No clinical conversion (N = 16) | P-value |
|---|---|---|---|
| > 9 lesions in T2 mode | 4 (80%) | 14 (87.5%) | 1.000 |
| > 1 lesion with gadolinium enhancement | 3 (60%) | 3 (18.75%) | 0.115 |
| > 1 medullary lesion | 2 (40%) | 1 (6.25%) | 0.128 |
| > 1 infratentorial lesion | 0 (0%) | 2 (12.5%) | 1.000 |
| > 1 lesion in T1 mode | 3 (60%) | 13 (81.25%) | 0.553 |
Fisher's exact test was employed for statistical analysis. Data presented in absolute frequencies and percentages; N: number of patients. This table illustrates differences in baseline nuclear magnetic resonance results between patients who clinically converted and those who did not at the 24-month follow-up.
In a univariate logistic regression analysis, we assessed clinical and neuroradiological variables at the onset of RIS diagnosis that could be associated with an increased risk of a first clinical event at the 2-year follow-up (Table 5). A younger age at the time of diagnosis, specifically less than 37 years, was a clear prognostic factor for the occurrence of a first clinical event [OR = 17.333 (95% CI 1.387–216.602), P = 0.025]. However, patient gender [OR = 0.100 (95% CI 0.07–1.490), P = 0.095] and the presence of pathological cerebrospinal fluid [OR = 1.000 (95% CI 0.108–9.229), P = 1.000] did not show a significant association. The presence of headaches, the primary reason for patient consultation, was not identified as a risk factor for disease development [OR = 0.400 (95% CI 0.051–3.125), P = 0.382]. Additionally, lesion burden in T2 mode [OR = 1.750 (95% CI 0.124–24.650), P = 0.678], spinal lesions [OR = 0.100 (95% CI 0.007–1.490), P = 0.095], and lesions in T1 mode [OR = 2.889 (95% CI 0.325–25.702), P = 0.341] were not associated with a subsequent clinical event. Three out of the 5 patients with Isolated Radiological Syndrome who clinically converted at the 24-month follow-up had gadolinium-enhancing lesions on the initial MRI. However, this factor was not associated with a higher risk of conversion to a first clinical event [OR = 0.154 (95% CI 0.017–1.369), P = 0.093].
Table 5.
Univariate Logistic regression analysis. Relative risk of clinical conversion in our RIS patients at 24 months of follow-up, according to different clinical and neuroradiological variables.
| Clinical and neuroradiological variables | OR | 95% CI | P |
|---|---|---|---|
| Age at diagnosis: < 37 years | 17.333 | (1.387–216.602) | 0.025 |
| Gender | 0.100 | (0.07–1.490) | 0.095 |
| Headaches | 0.400 | (0.051–3.125) | 0.382 |
| Pathological cerebrospinal fluid (OCB + IgG) | 1.000 | (0.108–9.229) | 1.000 |
| NMR: > 9 lesions in T2 mode | 1.750 | (0.124–24.650) | 0.678 |
| NMR: > 1 lesion in T1 mode | 2.889 | (0.325–25.702) | 0.341 |
| NMR: > 1 spinal cord lesion | 0.100 | (0.007–1.490) | 0.095 |
| NMR: > 1 gadolinium-enhancing lesion | 0.154 | (0.017–1.369) | 0.093 |
OR Regression coefficient, CI Confidence Interval, CSF Cerebrospinal Fluid, OCB Oligoclonal Bands, IgG Immunoglobulin G, NMR Nuclear Magnetic Resonance.
We also explored which type of defect in the signals of multifocal VEP at the onset of RIS diagnosis was associated with a higher relative risk of clinical conversion at the 2-year follow-up through univariate logistic regression analysis. The identified factors were a decrease in interocular amplitude [OR = 0.036, (95% CI 0.003–0.503); P = 0.014], a decrease in monocular amplitude [OR = 0.083, (95% CI 0.007–0.982); P = 0.048], and a prolonged interocular latency [OR = 0.095, (95% CI 0.009–0.972); P = 0.047]. No statistically significant differences were found in the interocular mean latency and monocular mean latency of aggregated responses from the 60 sectors of baseline multifocal fVEP in patients with RIS who clinically converted and those who did not convert at the 24-month follow-up (Mann–Whitney U test, P > 0.05) (Table 6). In summary, the logistic regression analysis revealed that specific defects in multifocal VEP signals at the initial diagnosis of RIS were associated with a higher relative risk of clinical conversion at the 2-year follow-up, including decreased interocular amplitude, decreased monocular amplitude, and elongated interocular latency.
Table 6.
Univariate logistic regression analysis. Relative risk of clinical conversion in our RIS patients at 24 months of follow-up, according to the amplitude and latency defects identified with the Multifocal VEP.
| Multifocal VEP | Odds ratio (OR) | 95% confidence interval (CI) | P-value |
|---|---|---|---|
| Amplitude (interocular probability plot) | 0.036 | (0.003–0.503) | 0.014 |
| Amplitude (monocular probability plot) | 0.083 | (0.007–0.982) | 0.048 |
| Latency (interocular probability plot) | 0.095 | (0.009–0.972) | 0.047 |
| Latency (monocular probability plot) | 0.346 | (0.039–3.080) | 0.341 |
| Mean latency (interocular) | 1.269 | (0.964–1.670) | 0.090 |
| Mean latency (monocular) | 1.153 | (0.966–1.376) | 0.114 |
Discussion
This is the first study to functionally assess the afferent visual pathway using the multifocal VEP in patients with RIS. The findings of this work are significant given the current interest in understanding the physiopathological mechanisms associated with demyelinating lesions in the early stages of MS. Our results suggest that neuroaxonal damage in the afferent visual pathway is present in these early stages of MS.
In our study, we demonstrated that our patients exhibited significant alterations in the interocular and monocular analysis of amplitudes (47.6%) of electrical signals from multifocal VEP, suggesting the presence of subclinical axonal degeneration processes in the afferent visual pathway in RIS. Changes in the amplitudes of multifocal VEP are a highly sensitive biomarker of the functional integrity of visual pathway axons14.
Additionally, our patients showed a significant delay in the interocular and monocular analysis of latencies (38%) of signals from multifocal VEP, suggesting the early presence of demyelination processes in the afferent visual pathway in RIS. Myelin plays a crucial role in trophic support and protects axons against the aggression of inflammatory mediators and immune cells in MS15. These defects in myelin sheaths are initially subclinical but expose axons, making them more vulnerable to degenerative processes. Delayed latencies of signals from VEP are a good indicator of demyelination processes in the visual pathway, and their severity correlates with the extent of elongation of bioelectrical signal latencies5–7. Changes in latencies often do not associate with any obvious clinical visual dysfunction in these early phases. In lesions of the afferent visual pathway, we observe concurrent phenomena of demyelination and axonal degeneration, and it can be challenging to separate the effect of both processes on VEP signals. Normalization of latency values over time indicates the effect of active remyelination processes in the visual system. Amplitudes can also recover in these early stages as conduction is unblocked, provided that axons are not damaged8. Experimental evidence suggests that a significant proportion of ganglion cell axons can be critically damaged in these early episodes of subclinical acute inflammation in the visual pathway9,10. This axonal damage will degenerate slowly and progressively, both retrogradely and anterogradely, and sometimes, this axonal damage takes a considerable time to manifest clinically11. Multifocal VEP technology has the advantage of early identification of functional defects in the afferent visual pathway before they are clinically observed or detected by other structural or psychophysical diagnostic techniques12.
Very few studies have assessed the afferent visual pathway with conventional Visual Evoked Potentials in patients with RIS, and none have used multifocal technology16,17. Gabelic et al.18 observed a significant elongation of latencies of conventional VEP responses in 14% (4/24) of their RIS patient cohort. Another prospective study with RIS patients did not yield conclusive data due to the small sample size19. Similarly, in a multicenter and longitudinal study, Lebrun et al.20 observed altered conventional VEP in 53% of patients with RIS. In this study, conventional VEP were associated with the number of hyperintense lesions in T2 mode and gadolinium-enhancing lesions on MRI, as well as pathological cerebrospinal fluid, serving as a predictive factor for clinical progression to MS. However, in a smaller study with 20 RIS patients, Knier et al.21 did not observe any abnormalities in conventional VEPs. Our results closely align with those observed by Lebrun et al.20 where the evaluation of multifocal VEP in two thirds of our RIS patients revealed abnormalities at the baseline. It is interesting to note that studies using conventional VEP exhibited considerable variability in results, potentially attributed to various factors: (1) in our clinical experience, very few centers conduct high-quality visual neurophysiology tests following ISCEV protocols, as evident in the graphs and tables published in these articles; (2) the studied patient cohorts were relatively small and included individuals with different risk profiles.
The multifocal VEP has demonstrated a generally good correlation with conventional VEP. However, the multifocal technology offers a distinct advantage by simultaneously and independently stimulating and analyzing multiple regions of the visual field. This capability enables the identification of subtle alterations that may go unnoticed by the aggregated single signal of cVEP22. Using the multifocal VEP, Pérez-Rico et al. 23 observed abnormal amplitudes and latencies in 48% and 23%, respectively, of patients with CIS. Similarly, Blanco et al. 12 have found anomalous amplitudes and latencies in 70% and 74%, respectively, of MS patients, regardless of a history of optic neuritis. Klistorner et al.24 have also demonstrated a correlation between the P100 wave of conventional and multifocal VEP in patients with optic neuritis. The multifocal VEP can detect a greater number of abnormal responses and have higher diagnostic sensitivity for detecting small peripheral lesions and defects in the upper visual field. Similarly, Laron et al.25 found that multifocal VEP exhibit greater sensitivity in identifying minor visual field abnormalities compared to automated static perimetry and optical coherence tomography in MS, regardless of whether they have a history of optic neuritis.
At the 24-month follow-up, 23.8% (5/21) of our RIS patient cohort clinically converted to either CIS or MS. These findings align with published data26–30. Recent studies have indicated that two-thirds of patients diagnosed with RIS will exhibit radiological progression, and one-third will experience a symptomatic first demyelinating neurological event, either CIS or MS, within the initial 5 years of follow-up27. By the 10-year mark, half of patients diagnosed with RIS will transition to advanced forms of the demyelinating process20,31. Notably, 12% of RIS patients will progress to primary progressive MS, more frequently observed in males, at older ages, and with a higher number of spinal cord lesions on MRI26.
Several factors have been associated with an increased risk of developing a first clinical event in RIS, including younger age at diagnosis, presence of spinal cord lesions 27,32, high lesion burden in T2 mode33, gadolinium-enhancing lesions32, and infratentorial lesions on MRI, along with the presence of oligoclonal bands and/or elevated immunoglobulin G index in cerebrospinal fluid32–35. Additionally, altered conventional VEP have been linked to an elevated risk of clinical conversion7.
In our study, the reduction in amplitudes and latency delay of multifocal VEP responses in patients with RIS were significantly associated with an elevated risk of conversion to CIS or MS at the 2-year follow-up. Additionally, within our RIS patient group, a diagnosis age younger than 37 years was significantly associated with a higher risk of clinical conversion to CIS or MS at the 2-year follow-up. However, we did not observe associations between variables from MRI or cerebrospinal fluid and the risk of clinical conversion, contrary to findings in previous studies37. This lack of observation was expected, given that our sample was not optimized to detect these associations, nor was it the primary focus of this study.
This study is subject to several limitations that warrant discussion. Firstly, the cohort of patients with RIS recruited for this study was relatively small. Despite this, the sample proved sufficient to detect significant changes in multifocal VEP in RIS, correlating with an increased risk of conversion to MS. However, the sample size was inadequate to identify changes in MRI. Addressing this limitation necessitates larger patient cohorts, prompting the consideration of multicenter studies. Given the challenge of recruiting an ample number of RIS patients within a reasonable timeframe at a single hospital center, multicenter collaboration becomes essential33–36.
Moreover, the follow-up duration for patients in this study was limited to a relatively short 2-year period. While this timeframe sufficed to observe changes in mfVEP results, the natural history of this disease suggests that an extended follow-up may reveal more conversions. Studies with follow-up periods of 5–10 years are currently under consideration for RIS. Unfortunately, due to the unavailability of specific image analysis software at that time, we were unable to conduct volumetric analysis of lesions observed in the MRI at our hospital's Radiology Department. Such volumetric analysis would have allowed for the assessment of cerebral atrophy and quantitative characteristics of MRI-observed lesions32–35.
The statistical analysis in this study involved selecting one eye per patient. Although some studies with small population samples choose to include both eyes, this approach requires more complex statistical techniques such as generalized estimating equations. There are additional neurological variables that were not explored in our study but could be valuable in future investigations. Notably, cognitive disorders have been found to be common in RIS patients, impacting neuropsychological functions such as memory, complex attention, information processing speed, and executive functions36.
Furthermore, the technology of multifocal VEP is not widely adopted in clinical practice within our environment. For many years, our center was the sole facility equipped with multifocal VEP equipment in our country. Conducting these recordings demands time and specialized knowledge. Nevertheless, in recent years, several commercial visual neurophysiology teams have integrated the option to perform multifocal VEP alongside conventional potentials, enhancing clinical experience with this technology.
In summary, the observed abnormalities in amplitudes and latencies of responses in multifocal VEP in RIS subjects indicate frequent and early involvement of the afferent visual pathway in the preclinical stages of MS. This suggests that multifocal VEP could emerge as a particularly useful tool in clinical practice for the diagnosis and objective monitoring of demyelinating processes in their early phases. Studying the afferent visual pathway through multifocal VEP in patients with RIS provides an opportunity to uncover etiopathogenic mechanisms of this disease before the onset of clinical signs. This represents a novel diagnostic technique capable of detecting axonal and/or demyelinating lesions in the optic nerve during the preclinical stages of MS.
Methods
This clinical, observational, longitudinal, and prospective study included 21 patients with RIS diagnosed at the Demyelinating Diseases Unit of the Neurology Department at Príncipe de Asturias University Hospital (Madrid, Spain). The study received approval from the Biomedical Research Ethics Committee of Príncipe de Asturias University Hospital and the Research Ethics Committee of the University of Alcalá. All data were handled in accordance with Regulation (EU) 2016/679, the General Data Protection Regulation (GDPR), and Organic Law 3/2018 on Personal Data Protection and Digital Rights Guarantee. Selected patients provided written consent after being informed about the study's purpose, the diagnostic tests involved, and the follow-up duration. Patients were made aware that their participation would not influence the standard protocol for their pathology reviews or the therapeutic decisions made by their neurologist.
Following a detailed neurological examination during the initial visit, a cerebrospinal fluid study and a MRI scan were requested. Upon confirming the diagnosis of RIS and meeting the inclusion criteria, baseline diagnostic tests for multifocal VEP were conducted. Patients were reviewed every 6 months, with MRI scans at 12 and 24 months. This visit protocol could be adjusted if patients exhibited any changes in their clinical course. All patients diagnosed with RIS in the neurological consultation, who agreed to participate, had to meet the following inclusion criteria: (1) aged between 18 and 55 years; (2) absence of cognitive or mental deficits that could interfere with understanding the study protocol; (3) no history of neurological symptoms suggestive of MS; (4) presence of asymptomatic brain lesions in MRI, hyperintense lesions in T2 mode > 3 mm in diameter meeting Barkhof criteria; (5) no autoimmune, vascular, or metabolic diseases causing abnormalities in MRI; (6) no retinal pathology, glaucoma, cataracts, or amblyopia; (7) no treatment with drugs causing toxic neuropathy.
Neuroradiological studies were conducted using a Philips Gyroscan MRI Unit (Philips Medical Systems, Best, The Netherlands) operating at 1.5 Tesla with a surface antenna. The MRI protocol included proton density or T2-weighted sequences, Fluid Attenuated Inversion-Recovery (FLAIR) T2-weighted sequences, spin-echo T1-weighted sequences, and Double Inversion-Recovery (DIR) sequences with and without gadolinium application. The obtained images were evaluated to ensure they met the diagnostic criteria for RIS34.
Multifocal visual-evoked potential recordings
For electrophysiological studies, a Veris™ multifocal Visual Evoked Potentials recording device from Electro-Diagnostic Imaging (San Mateo, California, USA) was used. The software for analyzing multifocal VEP signals in this study was custom-developed by our laboratory in collaboration with the Department of Electronic Engineering at the University of Alcalá. As there is currently no standard protocol for acquiring and analyzing multifocal VEP responses, generic recommendations from the International Society for Clinical Electrophysiology of Vision (ISCEV) 37 were followed. We applied similar recommendations for multifocal VEP as those suggested for conventional VEP22.
Non-invasive capture of electrophysiological signals in the visual cortex was achieved using gold-coated surface electrodes (Grass Model F-E5GH, Astro-Med Inc., West Warwick, USA). Three main recording channels were established, each consisting of a reference and an active electrode. A specially designed target-shaped stimulus, divided into 60 concentric sectors, was used for multifocal VEP recordings. Each sector comprised 16 squares—8 black with luminance < 3 cd/m2 and 8 white with 200 cd/m2 luminance—resulting in a Michelson contrast of 99%15. The stimulation followed a predetermined mathematical pseudo-random sequence, with a frequency of 75 Hz and a 50% inversion probability. Multifocal VEP recordings were analyzed using custom MATLAB™ software (The Mathworks, Natick, Massachusetts, USA)38. Amplitude analysis of the signals involved calculating the signal-to-noise ratio resulting from the ratio of the root mean square (RMS) of the signal amplitude in the window interval (45–150 ms) to the root mean square of the noise window amplitude (325–430 ms)25,39. Interocular amplitude differences for each patient were calculated using the logarithm of the interocular ratio for each location, generating an interocular probability graph (interocular amplitude analysis). Values obtained for each eye were compared with the normative database of the system, resulting in a monocular probability graph (monocular amplitude analysis). Latency analysis involved determining the time delay of the best intercorrelation between the patient's eye responses and the normative database responses (monocular analysis) or responses from both eyes of the patient (interocular analysis), using the 'xcorr standard' function in MATLAB40,41. For our study, interocular and monocular amplitude and latency analyses of multifocal VEP were considered abnormal if they met the following criteria: (1) two or more contiguous abnormal sectors with statistical significance P < 0.01; (2) three contiguous altered sectors with statistical significance of P < 0.05, with at least one having a significance of P < 0.0138,42.
Statistical analysis
For the statistical assessment of this study, we employed the SPSS™ software version 25 (IBM, USA). One eye per patient was randomly selected for all statistical analyses conducted. In the univariate descriptive analysis, percentages were provided for qualitative variables. For quantitative variables, the median and interquartile range were presented as measures of central location and dispersion, respectively. Group comparisons for qualitative variables were conducted using either the Chi-square test or Fisher's exact test, depending on the appropriateness in each case. Additionally, binary logistic regression analysis was performed to examine which risk factors were associated with clinical conversion. Odds ratios were calculated and presented along with their 95% confidence intervals. Throughout the study, a significance level of P < 0.05 was consistently applied.
Acknowledgements
This study was funded by the Carlos III Health Institute and the European Regional Development Fund (ERDF, “A way to make Europe”) under the "Plan Estatal de Investigación Científica y Técnica y de Innovación" (FIS PI22-01588).
Author contributions
M.I.R., N.C. and R.B. wrote the main manuscript text. M.I.R. and N.C. prepared tables 1–6 and Fig. 1. C.S., M.C.P.R.,C.S.and L.A. did most of the clinical, and neurorradiological exams. M.I.R and R.B. did most of the neurophysiological recordings. All authors reviewed the manuscript.
Data availability
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request. Data are located in controlled access data storage at Alcalá University.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Henderson, A. P. D. et al. Early factors associated with axonal loss after optic neuritis. Ann. Neurol.70, 955–963 (2011). 10.1002/ana.22554 [DOI] [PubMed] [Google Scholar]
- 2.Klistorner, A. et al. Assessment of opicinumab in acute optic neuritis u/sing Multifocal Visual Evoked Potential. CNS Drugs.32, 1159–1171 (2018). 10.1007/s40263-018-0575-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klistorner, A. et al. Interrelationship of optical coherence tomography and multifocal visual-evoked potentials after optic neuritis. Invest. Ophthalmol. Vis. Sci.51, 2770–2777 (2010). 10.1167/iovs.09-4577 [DOI] [PubMed] [Google Scholar]
- 4.Halliday, A. M., McDonald, W. I. & Mushin, J. Delayed visual evoked response in optic neuritis. Lancet.1, 982–985 (1972). 10.1016/S0140-6736(72)91155-5 [DOI] [PubMed] [Google Scholar]
- 5.Bjartmar, C., Kinkel, R. P., Kidd, G., Rudick, R. A. & Trapp, B. D. Axonal loss in normal-appearing white matter in a patient with acute MS. Neurology.57, 1248–1252 (2001). 10.1212/WNL.57.7.1248 [DOI] [PubMed] [Google Scholar]
- 6.DeLuca, G. C., Williams, K., Evangelou, N., Ebers, G. C. & Esiri, M. M. The contribution of demyelination to axonal loss in multiple sclerosis. Brain.129, 1507–1516 (2006). 10.1093/brain/awl074 [DOI] [PubMed] [Google Scholar]
- 7.Barnett, M. H. & Prineas, J. W. Relapsing and remitting multiple sclerosis: Pathology of the newly forming lesion. Ann. Neurol.55, 458–468 (2004). 10.1002/ana.20016 [DOI] [PubMed] [Google Scholar]
- 8.Longbrake, E. E., Lancia, S., Tutlam, N., Trinkaus, K. & Naismith, R. T. Quantitative visual tests after poorly recovered optic neuritis due to multiple sclerosis. Mult. Scler. Relat. Disord.10, 198–203 (2016). 10.1016/j.msard.2016.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green, A. J., McQuaid, S., Hauser, S. L., Allen, I. V. & Lyness, R. Ocular pathology in multiple sclerosis: retinal atrophy and inflammation irrespective of disease duration. Brain.133, 1591–1601 (2010). 10.1093/brain/awq080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frohman, E. M., Racke, M. K. & Raine, C. S. Multiple sclerosis—The plaque and its pathogenesis. N. Engl. J. Med.354, 942–955 (2006). 10.1056/NEJMra052130 [DOI] [PubMed] [Google Scholar]
- 11.Sepulcre, J. et al. Diagnostic accuracy of retinal abnormalities in predicting disease activity in MS. Neurology.68, 1488–1494 (2007). 10.1212/01.wnl.0000260612.51849.ed [DOI] [PubMed] [Google Scholar]
- 12.Blanco, R. et al. Functional assessment of the visual pathway with multifocal visual evoked potentials, and their relationship with disability in patients with multiple sclerosis. Mult. Scler.20, 183–191 (2014). 10.1177/1352458513493683 [DOI] [PubMed] [Google Scholar]
- 13.Hood, D. C., Zhang, X. & Winn, B. J. Detecting glaucomatous damage with multifocal visual evoked potentials: How can a monocular test work?. J. Glaucoma.12, 3–15 (2003). 10.1097/00061198-200302000-00002 [DOI] [PubMed] [Google Scholar]
- 14.Halliday, A. M. & McDonald, W. I. Pathophysiology of demyelinating disease. Br. Med. Bull.33, 21–27 (1977). 10.1093/oxfordjournals.bmb.a071390 [DOI] [PubMed] [Google Scholar]
- 15.You, Y. et al. Progressive inner nuclear layer dysfunction in non-optic neuritis eyes in MS. Neurol. Neuroimmunol. Neuroinflamm.5(1), e427 (2018). 10.1212/NXI.0000000000000427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lebrun, C. et al. Unexpected multiple sclerosis: Follow-up of 30 patients with magnetic resonance imaging and clinical conversion profile. J. Neurol. Neurosurg. Psychiatry.79, 195–198 (2008). 10.1136/jnnp.2006.108274 [DOI] [PubMed] [Google Scholar]
- 17.Siva, A. et al. Multiple sclerosis risk in radiologically uncovered asymptomatic possible inflammatory-demyelinating disease. Mult. Scler.15, 918–927 (2009). 10.1177/1352458509106214 [DOI] [PubMed] [Google Scholar]
- 18.Gabelić, T. et al. Differences in oligoclonal bands and visual evoked potentials in patients with radiologically and clinically isolated syndrome. Acta Neurol.113, 13–17 (2013). 10.1007/s13760-012-0106-1 [DOI] [PubMed] [Google Scholar]
- 19.Nakamura, M., Morris, M., Cerghet, M., Schultz, L. & Elias, S. Longitudinal Follow-up of a Cohort of patients with incidental abnormal magnetic resonance imaging findings at presentation and their risk of developing Multiple Sclerosis. Int Journal of MS Care.16, 111–115 (2014). 10.7224/1537-2073.2013-016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lebrun, C. et al. Association between clinical conversion to multiple sclerosis in radiologically isolated syndrome and magnetic resonance imaging, cerebrospinal fluid, and visual evoked potential: follow-up of 70 patients. Arch. Neurol.66, 841–846 (2009). 10.1001/archneurol.2009.119 [DOI] [PubMed] [Google Scholar]
- 21.Knier, B. et al. Optical coherence tomography indicates disease activity prior to clinical onset of central nervous system demyelination. Mult. Scler.22, 893–900 (2016). 10.1177/1352458515604496 [DOI] [PubMed] [Google Scholar]
- 22.Chan, H. L. et al. The detection of small relative simulated field defects using multifocal VEPs. Ophthalmic Physiol Opt.25, 224–232 (2005). 10.1111/j.1475-1313.2005.00273.x [DOI] [PubMed] [Google Scholar]
- 23.Pérez-Rico, C. et al. Evaluation of visual structural and functional factors that predict the development of multiple sclerosis in clinically isolated syndrome patients. Invest. Ophthalmol. Vis. Sci.55, 6127–6131 (2014). 10.1167/iovs.14-14807 [DOI] [PubMed] [Google Scholar]
- 24.Klistorner, A., Fraser, C., Garrick, R., Graham, S. & Arvind, H. Correlation between full-field and multifocal VEPs in optic neuritis. Doc Ophthalmol.116, 19–27 (2008). 10.1007/s10633-007-9072-y [DOI] [PubMed] [Google Scholar]
- 25.Laron, M. et al. Comparison of multifocal visual evoked potential, standard automated perimetry and optical coherence tomography in assessing visual. Mult. Scler.4, 412–426 (2010). 10.1177/1352458509359782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kantarci, O. H. et al. Primary progressive multiple sclerosis evolving from radiologically isolated syndrome. Ann. Neurol.79, 288–294 (2016). 10.1002/ana.24564 [DOI] [PubMed] [Google Scholar]
- 27.Okuda, D.T. et al. Radiologically Isolated Syndrome: 5-Year Risk for an Initial Clinical Event. PLoS ONE. 5, 9(3):e90509 (2014). [DOI] [PMC free article] [PubMed]
- 28.Lebrun, C. et al. Impact of pregnancy on conversion to clinically isolated syndrome in a radiologically isolated syndrome cohort. Mult. Scler.18, 1297–1302 (2012). 10.1177/1352458511435931 [DOI] [PubMed] [Google Scholar]
- 29.Okuda, D. T. et al. Asymptomatic spinal cord lesions predict disease progression in radiologically isolated syndrome. Neurology.76, 686–692 (2011). 10.1212/WNL.0b013e31820d8b1d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barkhof, F. Comparison of MRI criteria at first presentation to predict conversion to clinically definite multiple sclerosis. Brain.120, 2059–2069 (1997). 10.1093/brain/120.11.2059 [DOI] [PubMed] [Google Scholar]
- 31.Lebrun, C. et al. Radiologically Isolated Syndrome: 10-year risk estimate of a clinical event. Ann. Neurol.88, 407–417 (2020). 10.1002/ana.25799 [DOI] [PubMed] [Google Scholar]
- 32.Lebrun, C. et al. Risk factors and time to clinical symptoms of multiple sclerosis among patients with Radiologically Isolated Syndrome. JAMA Netw. Open.4(10), e2128271 (2021). 10.1001/jamanetworkopen.2021.28271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chaloulos-Iakovidis, P. et al. Predicting conversion to multiple sclerosis in patients with radiologically isolated syndrome: A retrospective study. Ther. Adv. Neurol. Disord.14, 1–10 (2021). 10.1177/17562864211030664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okuda, D.T. et al. Incidental MRI anomalies suggestive of multiple sclerosis. 72(9), 800–5 (2009) [DOI] [PubMed]
- 35.Matute-Blanch, C. et al. Neurofilament light chain and oligoclonal bands are prognostic biomarkers in radiologically isolated syndrome. Brain.141, 1085–1093 (2018). 10.1093/brain/awy021 [DOI] [PubMed] [Google Scholar]
- 36.Lebrun, C., Blanc, F., Brassat, D., Zephir, H. & de Seze, J. Cognitive function in radiologically isolated syndrome. Mult. Scler.16, 919–925 (2010). 10.1177/1352458510375707 [DOI] [PubMed] [Google Scholar]
- 37.Robson, A. G. et al. ISCEV guide to visual electrodiagnostic procedures. Doc. Ophthalmol.136, 1–26 (2018). 10.1007/s10633-017-9621-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baseler, H. A. & Sutter, E. E. M and P components of the VEP and their visual field distribution. Vision Res.37, 675–690 (1997). 10.1016/S0042-6989(96)00209-X [DOI] [PubMed] [Google Scholar]
- 39.Hood, D. C. et al. Determining abnormal interocular es of multifocal visual evoked potentials. Doc. Ophthalmol.109, 177–187 (2004). 10.1007/s10633-004-5511-1 [DOI] [PubMed] [Google Scholar]
- 40.Stellmann, J. P. et al. Pattern of gray matter volumes related to retinal thickness and its association with cognitive function in relapsing-remitting MS. Brain Behav.7, 1–14 (2017). 10.1002/brb3.614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saidha, S. et al. Primary retinal pathology in multiple sclerosis as detected by optical coherence tomography. Brain.134, 518–533 (2011). 10.1093/brain/awq346 [DOI] [PubMed] [Google Scholar]
- 42.Graham, S. L., Klistorner, A. I., Grigg, J. R. & Billson, F. A. Objective VEP perimetry in glaucoma: Asymmetry analysis to identify early deficits. J. Glaucoma.9, 10–19 (2000). 10.1097/00061198-200002000-00004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request. Data are located in controlled access data storage at Alcalá University.