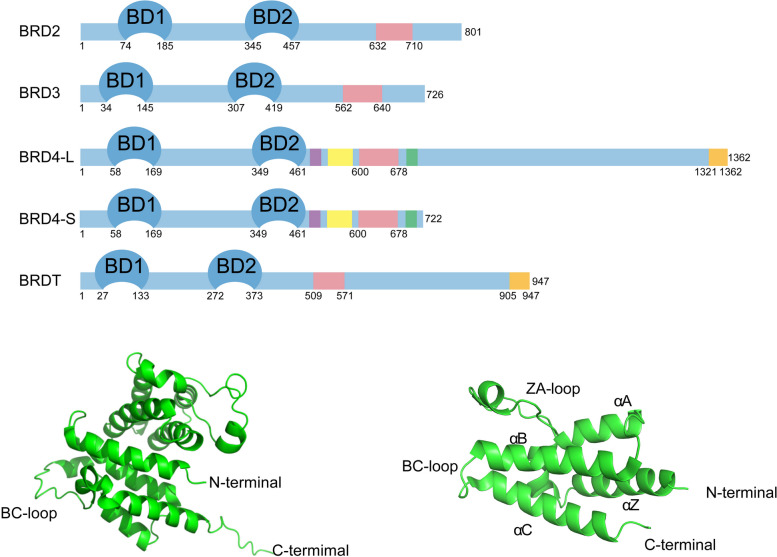
Fig. 2.
Structures of four members of the human bromodomain and extra-terminal structural domain (BET) family of proteins. All BET proteins have two tandem bromodomains (BD1, BD2) and an extra-terminal structural domain (ET), and the ET structural domains are indicated in pink. There are additional C-terminal domains (CTD), also known as positive transcription elongation factor b (P-TEFb) interaction domain (PID), in the long variant of BRD4 and in the BRDT proteins; CTD structural domains are indicated in orange. The short variant of BRD4 does not have CTD structural domains or histone acetyltransferases (HAT) kinase activity. Additionally, other structural domains were identified in the BRD4 protein, including the N-segment phosphorylation site (NPS), indicated in purple, with an amino acid range of 472–500; the basic residue-enriched structural domain, also known as the basic interaction structural domain (BID), indicated in yellow, with an amino acid range of 524–579; and the C-terminal phosphorylation site (CPS), indicated in green, with an amino acid range of 697–720. Among them, the bromodomain (BD1, BD2) mainly binds to acetylated lysine residues on histones and nonhistone proteins; the ET structural domain mainly mediates interactions with proteins such as transcription factors (TFs); the CTD is mainly responsible for the recruitment of and interaction with P-TEFb; and the NPS is negatively charged, which promotes BD2 binding to acetylated lysine residues, BID is positively charged and can form intramolecular contacts with NPS and inhibit BD2 binding to acetylated lysine residues