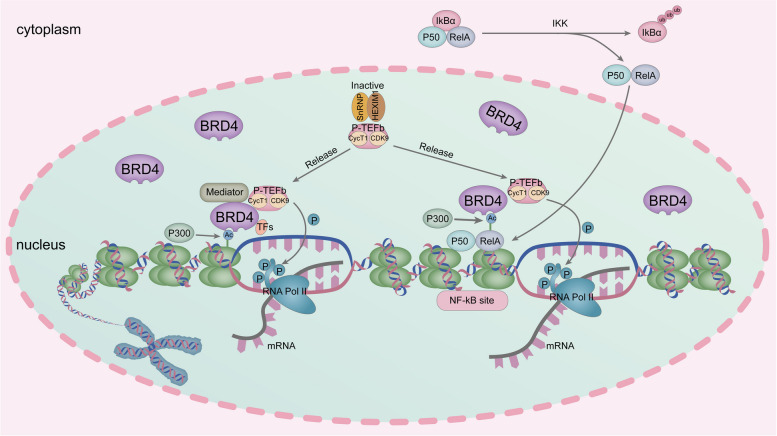
Fig. 3.
Schematic representation of the function of BRD4. In the presence of histone acetyltransferase (CBP/P300), lysine residues on histones are acetylated, and BRD4 binds to acetylated lysine residues and recruits transcription mediators (Mediator), TFs and P-TEFb. Then Ser2 of the RNA polymerase II (RNA Pol II) C-terminal motif (CTM) is phosphorylated via the cycle-dependent kinase 9 (CDK9) in P-TEFb, facilitating the transcription elongation of RNA Pol II. NF-kB consists of P50 and RelA dissociates from IkBα and translocates to the nucleus. Binds to the promoter of the target gene at the NF-kB site. In the presence of P300/CBP, the lysine at position 310 of RelA is acetylated. BRD4 binds to acetylated RelA and recruits P-TEFb. P-TEFb phosphorylates the CTM of RNA Pol II and promotes the transcription of NF-kB-related inflammatory genes