Abstract
Background
Previous studies have consistently reported a decrease in hospital admissions for respiratory diseases during the coronavirus disease 2019 (COVID-19) pandemic. However, the impact of the pandemic on idiopathic pulmonary fibrosis (IPF) admissions remains unknown.
Methods
This study used data from the Korean National Health Insurance Service database. IPF was defined based on the International Classification of Diseases 10th Revision (ICD-10) and rare intractable disease (RID) codes. The rate of IPF admissions was calculated by dividing the number of IPF admissions by the prevalence of IPF. The rate of IPF admissions during the COVID-19 pandemic (2020–2021) was compared with the mean rate of admissions during the prepandemic period (2017–2019) and presented as the rate ratio (RR). A sensitivity analysis was conducted on patients treated with systemic corticosteroids during IPF admission.
Results
In patients with IPF defined based on the ICD-10 (analysis 1), the RRs significantly decreased from March in 2020 to December 2021, except for June and September in 2020. Similarly, in patients with IPF defined based on the ICD-10 and RID (analysis 2), the RRs significantly decreased from March 2020 to December 2021, except for June and September 2020. In the sensitivity analysis of analysis 1, the RR significantly decreased in 2020 (0.93; 95%CI: 0.88–0.99; P = 0.029), whereas the RR in 2021 was not significantly different. The RRs in the sensitivity analysis of analysis 2 significantly decreased to 0.85 (0.79–0.92; P < 0.001) in 2020 and 0.82 (0.76–0.88; P < 0.001) in 2021. In the subgroup analysis, the rates of IPF admissions significantly decreased in 2020 and 2021 across both sexes, patients aged ≥ 60 years, and all household income groups.
Conclusions
The rate of IPF admissions significantly decreased during the COVID-19 pandemic. This result indicates that preventive measures against COVID-19 may effectively mitigate IPF exacerbation. Therefore, it is assumed that there is a close relationship between respiratory viral infections and IPF exacerbations.
Keywords: Idiopathic pulmonary fibrosis, COVID-19, Admission, Exacerbation
Background
The emergence of coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), in December 2019 has had a profound impact on global health [1]. In response to this pandemic, various policies, including vaccination, wearing face masks, the implementation of stringent measures, and the imposition of lockdowns, were implemented worldwide [2]. Lockdown emerged as a comprehensive policy aimed at curbing the spread of the virus by restricting gathering and interpersonal contact, effectively alleviating strain on healthcare facilities [3]. Unexpectedly, the implementation of lockdown affected mental and physical health and the management of chronic diseases [4, 5]. During the COVID-19 pandemic following the implementation of lockdown measures, the incidence of asthma exacerbations and hospitalization for chronic obstructive pulmonary disease (COPD) significantly decreased [6, 7]. During the pandemic, an unprecedented global decline was observed in the number of patients who developed influenza [8]. These observations suggest that preventive measures such as wearing face masks, physical distancing, and avoiding contact with others contribute to the reduction in the rate of asthma exacerbations and COPD [9].
Acute exacerbation of idiopathic pulmonary fibrosis (IPF) is a severe complication with a poor prognosis and high mortality rate. Acute exacerbation of IPF is classified as “triggered acute exacerbation” or “idiopathic acute exacerbation” according to the presence or absence of a known trigger [10]. Viral infections have been assumed to be significant triggers of acute exacerbations of IPF [11]. In this regard, case reports and series have indicated that COVID-19 can precipitate acute exacerbations in patients with IPF [12]. Underlying interstitial lung disease and lung fibrosis are known risk factors that increase morbidity and mortality in patients with COVID-19 [13]. However, comprehensive studies examining the trends in acute exacerbations of IPF during the COVID-19 pandemic at a nationwide level are lacking.
This study aimed to investigate whether the COVID-19 pandemic affected the hospital admission rates for IPF by comparing the admission rates before and during the pandemic.
Methods
Data source
Data from the Korea National Health Insurance Service (NHIS) was used in this retrospective cohort study. Established in 2002, this governmental health insurance program covers nearly all Korean citizens [14]. Healthcare providers from clinics, hospitals, and pharmacies in Korea submit medical service records to the NHIS for reimbursement. The NHIS adopted the International Classification of Diseases 10th revision (ICD-10) to categorize diseases based on diagnostic codes. The claims data comprised healthcare utilization information for both inpatients and outpatients, including patient demographics, diagnoses, procedures, and prescribed medications. The NHIS launched a rare intractable disease (RID) program in 2005, with IPF being listed as one of these diseases. Patients enrolled in the RID program receive an additional benefit, with 90% of the healthcare costs covered by the NHIS. Due to the rigorous verification process and reliability of RID registration, the RID registration database has been used in studies investigating the incidence and prevalence of other rare diseases [15–17].
Study participants
In the nationwide population, participants assigned the IPF diagnostic codes (J84.18) or RID codes (V236) at least once as a primary or first secondary diagnosis between January 2017 and December 2021 were included. Individuals assigned with the diagnostic code for connective tissue diseases (M05, M07, M30–35, M45) were excluded. Two definitions were used among the participants. The ICD-10 diagnostic code was solely used to identify patients with IPF in analysis 1. In analyses 2, IPF was diagnosed when both the diagnostic and RID codes were met. Comorbidities were determined based on the presence of the following diagnostic codes: coronary artery disease (I20–I25), heart failure (I110 and I50), cerebrovascular disease (G45, G46, I60–I69, and H340), diabetes (E10–E14), and chronic kidney disease (N18 and N19). Patients with COVID-19 were identified using the corresponding diagnostic codes (U07.1).
Study outcomes
IPF admission was defined as the presence of a corresponding diagnostic code at the primary or first secondary diagnosis in hospitalized patients. The number of IPF admissions in 2017–2021 for each year was determined. The prevalence of IPF in each year was defined as the total number of patients with an IPF diagnosis at least once in an outpatient clinic or hospital admission. The rate of IPF admissions was calculated by dividing the number of IPF admissions during each month by the prevalence of IPF in each corresponding year. The rate of IPF admission during the COVID-19 pandemic (2020–2021) was compared with the rate of admissions between 2017 and 2019. To ensure the robustness of our results across different clinical settings, sensitivity analysis and subgroup analysis were conducted.
Sensitivity analysis A assessed whether the IPF admission rate was affected by the number of patients with confirmed COVID-19. Therefore, patients with confirmed COVID-19 12 months before and during admission for IPF were excluded. In sensitivity analysis B, patients who were treated with systemic corticosteroid (methylprednisolone or prednisolone) during IPF admission were analyzed.
A subgroup analysis was conducted to determine whether the study results were influenced by patient characteristics. Variables included in the subgroup analysis were sex, age groups, and household income. The subjects of the subgroup analysis were limited to patients in analysis 2. Sensitivity analysis and subgroup analysis were assessed using yearly IPF admission rates, which were calculated by dividing the number of IPF admissions during a year by the prevalence of IPF in the corresponding year.
Statistical analysis
The baseline characteristics of the participants were compared using the Mann–Whitney U test for continuous variables and the chi-square test for categorical variables. Forecasting models for IPF were developed based on admission rates over the complete study period using an autoregressive integrated moving average (ARIMA) approach. The parameters for the ARIMA models were chosen utilizing the auto.arima function from the forecast package in R software [18]. The monthly hospitalization rates from January 2020 to December 2021 were visually compared against the 95% confidence intervals (CIs) of the forecasted values. The mean rate of admissions in 2017–2019 served as the reference. The rate of IPF admissions in 2020 and 2021 was compared with the reference and represented as rate ratios (RRs) with the corresponding 95% CIs. The RR was analyzed by dividing the year into 12 months to verify seasonal effects. All statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA). A P value of less than 0.05 was considered significant.
Results
Demographics of study participants
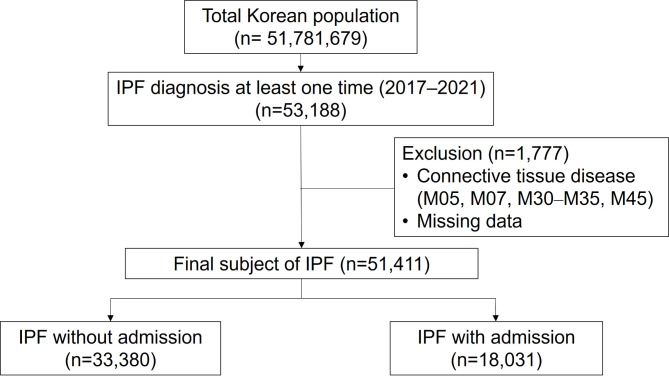
A total of 51,411 participants met the inclusion criteria. Among them, 33,380 participants were not admitted during the study period (2017–2021), while 18,031 participants were admitted at least once (Fig. 1). The proportion of men was significantly higher among patients admitted than among those who were not admitted (68.9% vs. 66.5%; P < 0.001). Patients who were admitted were significantly older, with a median age of 74.0 years, while those who were not admitted were aged 71.0 years (P < 0.001). All comorbidities, including coronary artery disease, heart failure, cerebrovascular disease, diabetes, and chronic kidney disease, were more prevalent in admitted patients than in those not admitted (Table 1).
Fig. 1.
Flowchart of the patient selection process
Table 1.
Baseline characteristics of the study participants
| Variables | Total | IPF without admission |
IPF with admission |
P value |
|---|---|---|---|---|
| Sex (male), n (%) | 34,609 (67.3) | 22,186 (66.5) | 12,423 (68.9) | < 0.001 |
| Age | ||||
| Median (Q1–Q3) | 72.0 (64.0–79.0) | 71.0 (62.0–78.0) | 74.0 (66.0–80.0) | < 0.001 |
| < 40 years | 908 (1.8) | 787 (2.4) | 121 (0.7) | < 0.001 |
| 40–49 years | 1,661 (3.2) | 1,369 (4.1) | 292 (1.6) | |
| 50–59 years | 5,371 (10.4) | 4,082 (12.2) | 1,289 (7.2) | |
| 60–69 years | 13,709 (26.7) | 9,213 (27.6) | 4,496 (24.9) | |
| ≥ 70 years | 29,762 (57.9) | 17,929 (53.7) | 11,833 (65.6) | |
| Conary artery disease | 5,343 (10.4) | 1,288 (3.9) | 4,055 (22.5) | < 0.001 |
| Heart failure | 6,687 (13.0) | 1,102 (3.3) | 5,585 (31.0) | < 0.001 |
| Cerebrovascular disease | 2,324 (4.5) | 371 (1.1) | 1,953 (10.8) | < 0.001 |
| Hypertension | 10,266 (20.0) | 2,365 (7.1) | 7,901 (43.8) | < 0.001 |
| Diabetes | 11,197 (21.8) | 2,668 (8.0) | 8,529 (47.3) | < 0.001 |
| Chronic kidney disease | 1,453 (2.8) | 386 (1.2) | 1,067 (5.9) | < 0.001 |
| Region of residence | ||||
| Urban | 13,269 (25.8) | 8,742 (26.2) | 4,527 (25.1) | 0.007 |
| Rural | 38,142 (74.2) | 24,638 (73.8) | 13,504 (74.9) | |
| Household income | ||||
| 1st quintile | 11,548 (22.5) | 7,368 (22.1) | 4,180 (23.2) | < 0.001 |
| 2nd quintile | 5,717 (11.1) | 3,853 (11.5) | 1,864 (10.3) | |
| 3rd quintile | 7,323 (14.2) | 4,742 (14.2) | 2,581 (14.3) | |
| 4th quintile | 9,829 (19.1) | 6,376 (19.1) | 3,453 (19.2) | |
| 5th quintile | 16,994 (33.1) | 11,041 (33.1) | 5,953 (33.0) |
Represented as n (%)
Rate of IPF admission
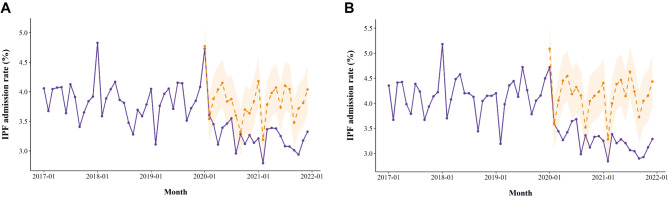
In analysis 1, hospitalizations for patients diagnosed with IPF showed a significant decline compared to the expected admission rates in 2020 and 2021 (Fig. 2A). The prevalence of IPF increased from 17,681 between 2017 and 2019 to 21,926 in 2021 (Table 2). The mean rate of IPF admissions in the January and February of reference year was 4.31% and 3.46%, respectively. No significant differences were observed in the admission rates during January and February of 2020: the RRs of IPF admission were 1.10 (95% CI: 1.00–1.22; P = 0.051) and 1.05 (0.94–1.17; P = 0.379), respectively. However, the rate of IPF admissions significantly decreased from March in 2020 to December 2021, except for June and September in 2020.
Fig. 2.
The monthly hospital admission rates for IPF diagnoses are shown in analysis 1 (A) and analysis 2 (B). The solid purple lines represent the actual admission rates during the study period, the dotted orange lines represent the predicted admission rates, and the orange shaded areas indicate the 95% confidence intervals of the predicted rates
Table 2.
Comparative analysis of the rate of IPF admissions in analysis 1
| Month | Year | Prevalence | Number of admissions | Admission rate (%) | Rate ratio (95% CI) |
P value |
|---|---|---|---|---|---|---|
| Jan | 2017–2019 | 17,681 | 762 | 4.31 | reference | |
| 2020 | 20,048 | 948 | 4.73 | 1.10 (1.00–1.22) | 0.051 | |
| 2021 | 21,926 | 704 | 3.21 | 0.74 (0.66–0.82) | < 0.001 | |
| Feb | 2017–2019 | 17,681 | 608 | 3.46 | reference | |
| 2020 | 20,048 | 723 | 3.61 | 1.05 (0.94–1.17) | 0.379 | |
| 2021 | 21,926 | 612 | 2.79 | 0.81 (0.72–0.90) | < 0.001 | |
| Mar | 2017–2019 | 17,681 | 687 | 3.90 | reference | |
| 2020 | 20,048 | 692 | 3.45 | 0.88 (0.79–0.98) | 0.025 | |
| 2021 | 21,926 | 738 | 3.37 | 0.86 (0.78–0.96) | 0.006 | |
| Apr | 2017–2019 | 17,681 | 711 | 4.03 | reference | |
| 2020 | 20,048 | 623 | 3.11 | 0.77 (0.69–0.85) | < 0.001 | |
| 2021 | 21,926 | 743 | 3.39 | 0.84 (0.75–0.93) | < 0.001 | |
| May | 2017–2019 | 17,681 | 725 | 4.10 | reference | |
| 2020 | 20,048 | 680 | 3.39 | 0.82 (0.74–0.91) | < 0.001 | |
| 2021 | 21,926 | 741 | 3.38 | 0.82 (0.74–0.91) | < 0.001 | |
| Jun | 2017–2019 | 17,681 | 661 | 3.74 | reference | |
| 2020 | 20,048 | 694 | 3.46 | 0.92 (0.83–1.03) | 0.149 | |
| 2021 | 21,926 | 713 | 3.25 | 0.87 (0.78–0.96) | 0.009 | |
| July | 2017–2019 | 17,681 | 713 | 4.03 | reference | |
| 2020 | 20,048 | 712 | 3.55 | 0.88 (0.79–0.97) | 0.014 | |
| 2021 | 21,926 | 675 | 3.08 | 0.76 (0.68–0.84) | < 0.001 | |
| Aug | 2017–2019 | 17,681 | 681 | 3.84 | reference | |
| 2020 | 20,048 | 593 | 2.96 | 0.76 (0.68–0.85) | < 0.001 | |
| 2021 | 21,926 | 674 | 3.07 | 0.79 (0.71–0.88) | < 0.001 | |
| Sep | 2017–2019 | 17,681 | 602 | 3.40 | reference | |
| 2020 | 20,048 | 658 | 3.28 | 0.96 (0.86–1.08) | 0.508 | |
| 2021 | 21,926 | 660 | 3.01 | 0.88 (0.79–0.99) | 0.026 | |
| Oct | 2017–2019 | 17,681 | 653 | 3.69 | reference | |
| 2020 | 20,048 | 625 | 3.12 | 0.84 (0.75–0.94) | 0.002 | |
| 2021 | 21,926 | 644 | 2.94 | 0.79 (0.71–0.88) | < 0.001 | |
| Nov | 2017–2019 | 17,681 | 665 | 3.76 | reference | |
| 2020 | 20,048 | 655 | 3.27 | 0.86 (0.77–0.96) | 0.009 | |
| 2021 | 21,926 | 696 | 3.17 | 0.84 (0.75–0.93) | 0.001 | |
| Dec | 2017–2019 | 17,681 | 696 | 3.93 | reference | |
| 2020 | 20,048 | 629 | 3.14 | 0.79 (0.71–0.88) | < 0.001 | |
| 2021 | 21,926 | 729 | 3.32 | 0.84 (0.76–0.93) | 0.001 |
In analysis 2, hospital admissions for patients diagnosed with IPF appeared a notable decrease compared to the predicted rates (Fig. 2B). The prevalence of IPF increased from 9,808 between 2017 and 2019 to 14,385 in 2021 (Table 3). The rate of IPF admissions showed a similar trend compared to analysis 1: the rate of IPF admissions significantly decreased from March 2020 to the end of the study period, except for June and September 2020.
Table 3.
Comparative analysis of the rate of IPF admissions in analysis 2
| Month | Year | Prevalence | Number of admissions | Admission rate (%) | Rate ratio (95% CI) |
P value |
|---|---|---|---|---|---|---|
| Jan | 2017–2019 | 9,808 | 448 | 4.58 | reference | |
| 2020 | 12,732 | 601 | 4.72 | 1.04 (0.91–1.17) | 0.588 | |
| 2021 | 14,385 | 468 | 3.25 | 0.70 (0.62–0.80) | < 0.001 | |
| Feb | 2017–2019 | 9,808 | 343 | 3.52 | reference | |
| 2020 | 12,732 | 457 | 3.59 | 1.03 (0.89–1.18) | 0.710 | |
| 2021 | 14,385 | 409 | 2.84 | 0.81 (0.70–0.93) | 0.004 | |
| Mar | 2017–2019 | 9,808 | 406 | 4.16 | reference | |
| 2020 | 12,732 | 438 | 3.44 | 0.83 (0.72–0.95) | 0.006 | |
| 2021 | 14,385 | 487 | 3.39 | 0.81 (0.71–0.93) | 0.002 | |
| Apr | 2017–2019 | 9,808 | 433 | 4.42 | reference | |
| 2020 | 12,732 | 416 | 3.27 | 0.73 (0.64–0.84) | < 0.001 | |
| 2021 | 14,385 | 461 | 3.20 | 0.72 (0.63–0.82) | < 0.001 | |
| May | 2017–2019 | 9,808 | 427 | 4.33 | reference | |
| 2020 | 12,732 | 436 | 3.42 | 0.78 (0.68–0.89) | < 0.001 | |
| 2021 | 14,385 | 472 | 3.28 | 0.75 (0.65–0.85) | < 0.001 | |
| Jun | 2017–2019 | 9,808 | 398 | 4.04 | reference | |
| 2020 | 12,732 | 464 | 3.64 | 0.89 (0.78–1.03) | 0.109 | |
| 2021 | 14,385 | 461 | 3.20 | 0.78 (0.68–0.90) | < 0.001 | |
| July | 2017–2019 | 9,808 | 437 | 4.44 | reference | |
| 2020 | 12,732 | 469 | 3.68 | 0.82 (0.72–0.94) | 0.003 | |
| 2021 | 14,385 | 441 | 3.07 | 0.68 (0.59–0.78) | < 0.001 | |
| Aug | 2017–2019 | 9,808 | 413 | 4.21 | reference | |
| 2020 | 12,732 | 380 | 2.98 | 0.70 (0.61–0.81) | < 0.001 | |
| 2021 | 14,385 | 438 | 3.04 | 0.71 (0.62–0.82) | < 0.001 | |
| Sep | 2017–2019 | 9,808 | 357 | 3.63 | reference | |
| 2020 | 12,732 | 428 | 3.36 | 0.92 (0.80–1.06) | 0.259 | |
| 2021 | 14,385 | 417 | 2.90 | 0.79 (0.68–0.91) | 0.001 | |
| Oct | 2017–2019 | 9,808 | 394 | 4.01 | reference | |
| 2020 | 12,732 | 397 | 3.12 | 0.77 (0.67–0.89) | < 0.001 | |
| 2021 | 14,385 | 421 | 2.93 | 0.72 (0.63–0.83) | < 0.001 | |
| Nov | 2017–2019 | 9,808 | 407 | 4.15 | reference | |
| 2020 | 12,732 | 424 | 3.33 | 0.80 (0.69–0.91) | 0.001 | |
| 2021 | 14,385 | 449 | 3.12 | 0.74 (0.65–0.85) | < 0.001 | |
| Dec | 2017–2019 | 9,808 | 422 | 4.29 | reference | |
| 2020 | 12,732 | 426 | 3.35 | 0.77 (0.67–0.88) | < 0.001 | |
| 2021 | 14,385 | 473 | 3.29 | 0.76 (0.66–0.86) | < 0.001 |
Sensitivity analysis
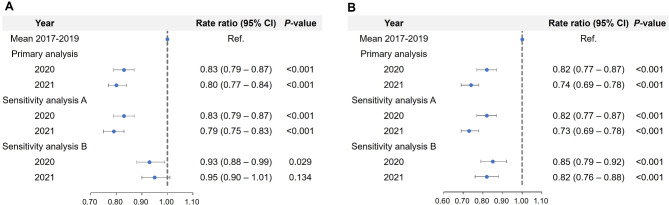
In analysis 1, 9 patients (0.20%) in 2020 and 46 patients (0.94%) in 2021 were confirmed with COVID-19 before 1year of hospitalization. When those with COVID-19 were excluded, the results of the primary analysis remained unchanged in sensitivity analysis A (Fig. 3A). In sensitivity analysis B, RR was significantly decreased in 2020 (0.93; 0.88–0.99; P = 0.029), whereas RR in 2021 was not different compared to 2017–2021 (0.95; 0.90–1.01; P = 0.134).
Fig. 3.
Rate ratio of IPF admissions in different outcome settings of patients in analysis 1 (A) and analysis 2 (B)
In analysis 2, 6 patients (0.20%) in 2020 and 21 patients (0.67%) 2021 were confirmed with COVID-19 before 1year of hospitalization. Significantly lower RRs were identified in sensitivity analysis A, which were similar to those in the primary analysis. (Fig. 3B). The RRs of IPF admissions in sensitivity analysis B were significantly decreased to 0.85 (0.79–0.92; P < 0.001) in 2020 and 0.82 (0.76–0.88; P < 0.001) in 2021.
Subgroup analysis
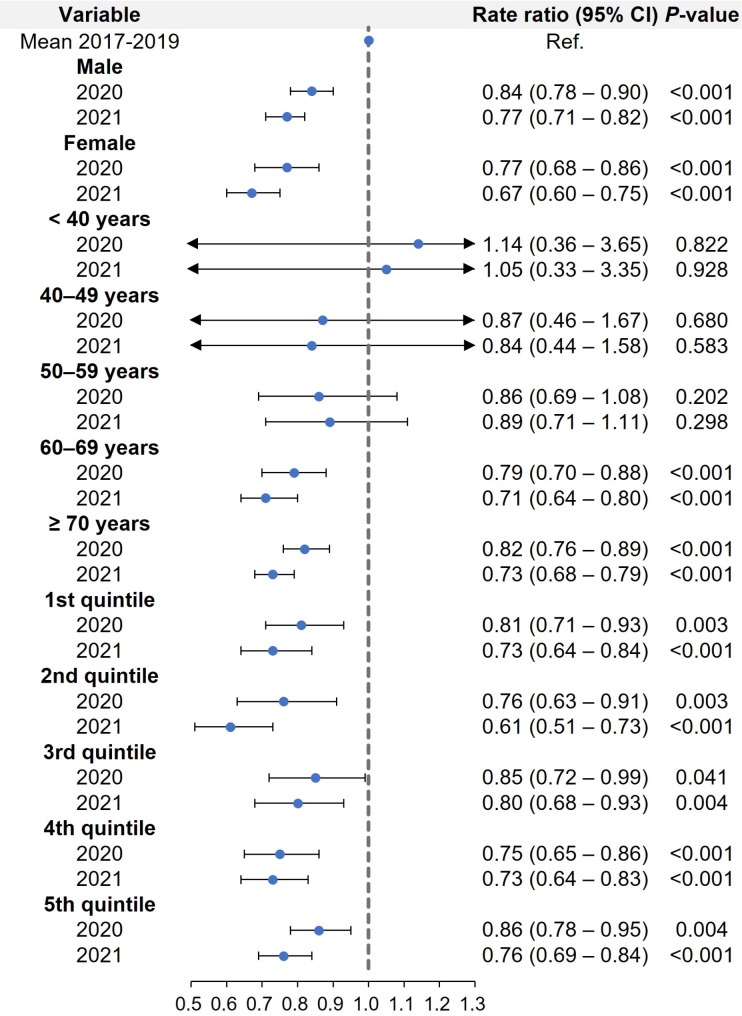
The rates of IPF admissions significantly decreased in 2020 and 2021, regardless of sex (Fig. 4). In the baseline characteristics, elderly patients were predominant: the proportions of the age groups < 60 years and ≥ 60 years were 15.4% and 84.6%, respectively. The RRs of patients under 60 years old did not differ between the prepandemic (2017–2019) and the pandemic period (2020–2021). However, patients aged 60 years and older showed a significantly lower rate of admission for IPF in 2020 and 2021. All household income groups exhibited significantly lower rates of IPF admissions in 2020 and 2021 compared to 2017–2019.
Fig. 4.
Rate ratio of IPF admissions in subgroup analysis of patients in analysis 2: sex, age groups, and household income
Discussion
The rate of IPF admissions significantly decreased during the COVID-19 pandemic compared to the prepandemic era. We obtained consistent results for different definitions of patients with IPF, various study periods, and admission criteria in the sensitivity analyses. Subgroup analysis also indicated the robustness of study results. To the best of our knowledge, this population-based study is the first to examine the trends in IPF admissions during the COVID-19 pandemic.
Findings from previous studies suggest that acute exacerbation of chronic airway disease triggered by respiratory viral infection may be preventable. This conclusion is supported by research comparing the incidence of exacerbations between the COVID-19 pandemic and prepandemic periods. One cohort study reported a 54% decrease in the mean number of exacerbations of COPD and a 45% decrease in the number of patients experiencing exacerbations of COPD [19]. Another multicenter study reported a 50% decrease in the number of admissions for respiratory viral infection-associated acute exacerbation of COPD during the COVID-19 pandemic compared with the preceding 2-year prepandemic period [20]. A previous meta-analysis reported that an RR of admission of 0.50 (95% CI: 0.44–0.57) for COPD during the COVID-19 pandemic compared with the prepandemic period [21]. Additionally, the positivity rate of respiratory specimens for influenza sharply dropped from approximately 20% during the prepandemic period to 2% during the COVID-19 pandemic [22]. Therefore, we hypothesize that preventive measures against COVID-19 may be also effective in mitigating IPF exacerbation.
During the COVID-19 pandemic, several factors may have contributed to the decreased hospitalization rates among patients with IPF. Notably, implementation of social distancing measures, the avoidance of hospitals, and reduced air pollution levels stand out as prominent among these factors [2]. Implementing social distancing measures during the pandemic played a critical role in reducing the spread of infectious diseases, including respiratory infections that can exacerbate IPF. Studies have shown that these public health interventions led to a significant decline in the incidence of respiratory infections [23]. The widespread fear of contracting COVID-19 influenced the behavior of individuals with critical conditions such as IPF. Patients were likely to avoid elective procedures to minimize their risk of exposure to the virus. The pandemic-related reductions in air pollution levels might have positively impacted respiratory health, particularly for those with chronic lung diseases like IPF [24]. During the pandemic, many regions experienced decreased industrial activity and vehicular traffic, leading to lower levels of air pollutants such as nitrogen dioxide and particulate matter [25]. Research indicates that lower air pollution levels were associated with fewer respiratory problems and hospitalizations during this period [26]. For IPF patients, cleaner air likely translated into better overall respiratory health and reduced hospital admissions [27].
The relationship between IPF and viral infections has been examined in two recent meta-analyses. Sheng et al. reported that chronic viral infections such as Epstein-Barr virus, cytomegalovirus, human herpesvirus 7, and human herpesvirus 8 were associated with an elevated risk of developing IPF (odds ratio [OR]: 3.48; 95% CI: 1.61–7.52) [28]. Mostafaei et al. conducted a meta-analysis reporting viral infection prevalence of 53.7% (95% CI 38.1–69.1%) in IPF [29]. These meta-analyses indicate that viral infection might be involved in the pathogenesis of IPF. However, establishing a causal relationship between viral infection and IPF requires validation with robust clinical evidence, as many confounding factors were not adjusted in the statistical methods of meta-analysis [30]. Meanwhile, viral infection was not associated with the exacerbation of IPF in a meta-analysis (OR: 0.99; 95% CI: 0.47–2.12) [28]. Although our study did not assess viral infection with objective measurements, the results indicated that viral infection could act as a triggering factor for the exacerbation of IPF, which is an inconsistent finding with meta-analysis. Further, studies are required to investigate the potential role of viral infections in the pathogenesis and exacerbation of IPF.
COVID-19 is associated with pulmonary fibrosis. Numerous studies have reported that a substantial proportion of patients infected with SARS-CoV-2 develop pulmonary fibrosis [31]. Nearly 15% of hospitalized patients with COVID-19 exhibited features of acute respiratory distress syndrome, which predisposes individuals to pulmonary fibrosis [32]. The common risk factors shared between severe COVID-19 and IPF, such as smoking, older age, genetic predisposition, male sex, and comorbidities, suggest an increased susceptibility to severe COVID-19 among patients with IPF [33]. Although it remains unclear whether patients with IPF are more susceptible to COVID-19 compared with those without IPF, relevant studies indicate that key molecules mediating SARS-CoV-2 cell entry, namely, angiotensin-converting enzyme 2 and TMPRSS2, were significantly upregulated in the fibroblasts of patients with IPF, potentially contributing to their increased susceptibility to COVID-19 [32]. In sensitivity analysis A of our study, we tried to assess the role of COVID-19 in the admission rate of IPF. However, only 55 patients with IPF were confirmed to have COVID-19, which did not significantly affect the IPF admission rate during the COVID-19 pandemic. Further clinical studies are warranted to determine the impact of COVID-19 on the prognosis of patients with IPF.
Our results identified a further increased prevalence of IPF during the COVID-19 pandemic. The global incidence and prevalence of IPF have been increasing since before the COVID-19 pandemic [34]. According to previous studies, the global prevalence rates of IPF range between 0.33 and 2.51 (per 10,000 individuals) in Europe and 2.40 and 2.98 in North America [35]. In Korea, the prevalence of IPF is high at 4.51, surpassing the criteria for a rare disease. A previous study investigated the incidence of IPF over time in Korea [36]. IPF was identified based on the ICD-10 and RID codes in the NHIS database, consistent with the definition of IPF in our study. The incidence of IPF increased from 3.56 in 2011 to 7.91 in 2019 per 100,000 individuals. Enhanced diagnostic technologies, increased awareness about IPF, and the availability of effective treatments such as pirfenidone and nintedanib may contribute to the rising diagnosis rate of this condition [35]. Therefore, this increasing trend of IPF prevalence may persist and contribute to the decreasing rate of IPF admissions during the COVID-19 pandemic.
The strength of our study lies in the robustness of the results. We assessed the trend of IPF admissions during the COVID-19 pandemic compared with the COVID-19 prepandemic period using different definitions of IPF, outcomes, and study periods. Most studies investigating the trends in certain diseases during the COVID-19 pandemic have limited their study period to the first half of 2020 [21, 37]. However, we extended the study period to include the second half of 2020 and 2021.
This study has several limitations. First, IPF was defined based solely on the diagnostic codes. Although ICD-10 and RID codes were used, well-established, and validated for diagnostic accuracy, the definition of IPF based solely on diagnostic codes has not yet been formally verified. Therefore, the IPF may have been overestimated or underestimated in this study. Second, the nature of hospital admission could not be distinguished. Patients admitted for IPF had diverse comorbidities with a higher prevalence compared with those who were not admitted, as represented in the baseline characteristics. Thus, we were unable to ascertain whether admission was directly related to the treatment of IPF or the associated comorbidities.
Conclusions
The rate of IPF admissions significantly decreased during the COVID-19 pandemic. This result indicates that preventive measures against COVID-19 may effectively mitigate IPF exacerbation. Thereforfe, it is assumed that there is a close relationship between respiratory viral infections and IPF exacerbations. Further studies are warranted to investigate the factors triggering IPF exacerbation and to elucidate the role of viral infection and COVID-19 in the development and exacerbation of IPF.
Acknowledgements
Not applicable.
Abbreviations
- COVID-19
Coronavirus disease 2019
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- COPD
Chronic obstructive pulmonary disease
- IPF
Idiopathic pulmonary fibrosis
- NHIS
National Health Insurance Service
- ICD-10
International Classification of Diseases 10th revision
- RID
Rare intractable disease
- RR
Rate ratio
- CI
Confidence interval
Author contributions
Conceptualization: JH Lee. Methodology: IS Cho, JH Lim. Validation: JH Lee. Formal analysis: IS Cho, JH Lim. Investigation: JH Lee. Data curation: IS Cho, JH Lim. Writing-original draft: IS Cho. Writing-review & editing: MS Chang. Visualization: JH Lim. Supervision: JH Lee. All authors reviewed the manuscript.
Funding
There was no funding for this research.
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of Wonju Severance Christian Hospital (CR322312) and adhered to the principles of the Declaration of Helsinki. Due to the retrospective nature of the study using anonymous claims data, the requirement for informed consent was waived.
Disclosure
All authors have no potential conflicts of interest.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
In-So Cho and Jihye Lim contributed equally to this work.
References
- 1.Nicola M, Alsafi Z, Sohrabi C, Kerwan A, Al-Jabir A, Iosifidis C, Agha M, Agha R. The socio-economic implications of the coronavirus pandemic (COVID-19): a review. Int J Surg. 2020;78:185–93. 10.1016/j.ijsu.2020.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen CWS, So MKP, Liu FC. Assessing government policies’ impact on the COVID-19 pandemic and elderly deaths in East Asia. Epidemiol Infect. 2022;150:e161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spiliopoulos L. On the effectiveness of COVID-19 restrictions and lockdowns: pan metron ariston. BMC Public Health. 2022;22(1):1842. 10.1186/s12889-022-14177-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saqib MAN, Siddiqui S, Qasim M, Jamil MA, Rafique I, Awan UA, Ahmad H, Afzal MS. Effect of COVID-19 lockdown on patients with chronic diseases. Diabetes Metab Syndr. 2020;14(6):1621–3. 10.1016/j.dsx.2020.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Onyeaka H, Anumudu CK, Al-Sharify ZT, Egele-Godswill E, Mbaegbu P. COVID-19 pandemic: a review of the global lockdown and its far-reaching effects. Sci Prog. 2021;104(2):368504211019854. 10.1177/00368504211019854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah SA, Quint JK, Nwaru BI, Sheikh A. Impact of COVID-19 national lockdown on asthma exacerbations: interrupted time-series analysis of English primary care data. Thorax. 2021;76(9):860–6. 10.1136/thoraxjnl-2020-216512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan KPF, Ma TF, Kwok WC, Leung JKC, Chiang KY, Ho JCM, Lam DCL, Tam TCC, Ip MSM, Ho PL. Significant reduction in hospital admissions for acute exacerbation of chronic obstructive pulmonary disease in Hong Kong during coronavirus disease 2019 pandemic. Respir Med. 2020;171:106085. 10.1016/j.rmed.2020.106085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubin R. Influenza’s unprecedented low profile during COVID-19 pandemic leaves experts wondering what this flu season has in store. JAMA. 2021;326(10):899–900. 10.1001/jama.2021.14131 [DOI] [PubMed] [Google Scholar]
- 9.Halpin DMG, Vogelmeier CF, Agusti A. COVID-19 and COPD: lessons beyond the pandemic. Am J Physiol Lung Cell Mol Physiol. 2021;321(5):L978–82. 10.1152/ajplung.00386.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collard HR, Ryerson CJ, Corte TJ, Jenkins G, Kondoh Y, Lederer DJ, Lee JS, Maher TM, Wells AU, Antoniou KM, et al. Acute exacerbation of idiopathic pulmonary fibrosis. An international working group report. Am J Respir Crit Care Med. 2016;194(3):265–75. 10.1164/rccm.201604-0801CI [DOI] [PubMed] [Google Scholar]
- 11.Wootton SC, Kim DS, Kondoh Y, Chen E, Lee JS, Song JW, Huh JW, Taniguchi H, Chiu C, Boushey H, et al. Viral infection in acute exacerbation of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183(12):1698–702. 10.1164/rccm.201010-1752OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goto Y, Sakamoto K, Fukihara J, Suzuki A, Omote N, Ando A, Shindo Y, Hashimoto N. COVID-19-triggered acute exacerbation of IPF, an underdiagnosed clinical entity with two-peaked respiratory failure: a case report and literature review. Front Med (Lausanne). 2022;9:815924. 10.3389/fmed.2022.815924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallay L, Uzunhan Y, Borie R, Lazor R, Rigaud P, Marchand-Adam S, Hirschi S, Israel-Biet D, Valentin V, Cottin V. Risk factors for mortality after COVID-19 in patients with preexisting interstitial lung disease. Am J Respir Crit Care Med. 2021;203(2):245–9. 10.1164/rccm.202007-2638LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheol Seong S, Kim YY, Khang YH, Heon Park J, Kang HJ, Lee H, Do CH, Song JS, Hyon Bang J, Ha S, et al. Data resource profile: the National Health Information Database of the National Health Insurance Service in South Korea. Int J Epidemiol. 2017;46(3):799–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim HJ, Hann HJ, Hong SN, Kim KH, Ahn IM, Song JY, Lee SH, Ahn HS. Incidence and natural course of inflammatory bowel disease in Korea, 2006–2012: a nationwide population-based study. Inflamm Bowel Dis. 2015;21(3):623–30. 10.1097/MIB.0000000000000313 [DOI] [PubMed] [Google Scholar]
- 16.Yoon HY, Kim HM, Kim YJ, Song JW. Prevalence and incidence of sarcoidosis in Korea: a nationwide population-based study. Respir Res. 2018;19(1):158. 10.1186/s12931-018-0871-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahn IM, Park DH, Hann HJ, Kim KH, Kim HJ, Ahn HS. Incidence, prevalence, and survival of moyamoya disease in Korea: a nationwide, population-based study. Stroke. 2014;45(4):1090–5. 10.1161/STROKEAHA.113.004273 [DOI] [PubMed] [Google Scholar]
- 18.Hyndman RJ, Khandakar Y. Automatic time series forecasting: the forecast package for R. J Stat Softw. 2008;27:1–22. 10.18637/jss.v027.i03 [DOI] [Google Scholar]
- 19.Trujillo C, Garnet B, Zadeh AV, Urdaneta G, Campos M. Decrease in exacerbations during the coronavirus disease 2019 pandemic in a cohort of veterans with COPD. Chronic Obstr Pulm Dis. 2021;8(4):572–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan JY, Conceicao EP, Wee LE, Sim XYJ, Venkatachalam I. COVID-19 public health measures: a reduction in hospital admissions for COPD exacerbations. Thorax. 2021;76(5):512–3. 10.1136/thoraxjnl-2020-216083 [DOI] [PubMed] [Google Scholar]
- 21.Alqahtani JS, Oyelade T, Aldhahir AM, Mendes RG, Alghamdi SM, Miravitlles M, Mandal S, Hurst JR. Reduction in hospitalised COPD exacerbations during COVID-19: a systematic review and meta-analysis. PLoS ONE. 2021;16(8):e0255659. 10.1371/journal.pone.0255659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olsen SJ, Azziz-Baumgartner E, Budd AP, Brammer L, Sullivan S, Pineda RF, Cohen C, Fry AM. Decreased influenza activity during the COVID-19 pandemic-United States, Australia, Chile, and South Africa, 2020. Am J Transpl. 2020;20(12):3681–5. 10.1111/ajt.16381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim MC, Kweon OJ, Lim YK, Choi SH, Chung JW, Lee MK. Impact of social distancing on the spread of common respiratory viruses during the coronavirus disease outbreak. PLoS ONE. 2021;16(6):e0252963. 10.1371/journal.pone.0252963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ravindra K, Singh T, Vardhan S, Shrivastava A, Singh S, Kumar P, Mor S. COVID-19 pandemic: what can we learn for better air quality and human health? J Infect Public Health. 2022;15(2):187–98. 10.1016/j.jiph.2021.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adam MG, Tran PTM, Balasubramanian R. Air quality changes in cities during the COVID-19 lockdown: a critical review. Atmos Res. 2021;264:105823. 10.1016/j.atmosres.2021.105823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong JY, Bang T, Kim SB, Hong M, Jung J. Atmosphere particulate matter and respiratory diseases during COVID-19 in Korea. Sci Rep. 2024;14(1):10074. 10.1038/s41598-024-59643-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Majewski S, Piotrowski WJ. Air Pollution-An overlooked risk factor for idiopathic pulmonary fibrosis. J Clin Med 2020, 10(1). [DOI] [PMC free article] [PubMed]
- 28.Sheng G, Chen P, Wei Y, Yue H, Chu J, Zhao J, Wang Y, Zhang W, Zhang HL. Viral infection increases the risk of idiopathic pulmonary fibrosis: a meta-analysis. Chest. 2020;157(5):1175–87. 10.1016/j.chest.2019.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mostafaei S, Sayad B, Azar MEF, Doroudian M, Hadifar S, Behrouzi A, Riahi P, Hussen BM, Bayat B, Nahand JS, et al. The role of viral and bacterial infections in the pathogenesis of IPF: a systematic review and meta-analysis. Respir Res. 2021;22(1):53. 10.1186/s12931-021-01650-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang AG. Viral infection and idiopathic pulmonary fibrosis risk: still need more evidence. Chest. 2020;157(6):1687–8. 10.1016/j.chest.2019.12.050 [DOI] [PubMed] [Google Scholar]
- 31.Ojha V, Mani A, Pandey NN, Sharma S, Kumar S. CT in coronavirus disease 2019 (COVID-19): a systematic review of chest CT findings in 4410 adult patients. Eur Radiol. 2020;30(11):6129–38. 10.1007/s00330-020-06975-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen H, Zhang N, Liu Y, Yang X, He Y, Li Q, Shen X, Zhu Y, Yang Y. The interaction between pulmonary fibrosis and COVID-19 and the application of related anti-fibrotic drugs. Front Pharmacol. 2021;12:805535. 10.3389/fphar.2021.805535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.George PM, Wells AU, Jenkins RG. Pulmonary fibrosis and COVID-19: the potential role for antifibrotic therapy. Lancet Respir Med. 2020;8(8):807–15. 10.1016/S2213-2600(20)30225-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pergolizzi JV Jr., LeQuang JA, Varrassi M, Breve F, Magnusson P, Varrassi G. What do we need to know about rising rates of idiopathic pulmonary fibrosis? A narrative review and update. Adv Ther. 2023;40(4):1334–46. 10.1007/s12325-022-02395-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maher TM, Bendstrup E, Dron L, Langley J, Smith G, Khalid JM, Patel H, Kreuter M. Global incidence and prevalence of idiopathic pulmonary fibrosis. Respir Res. 2021;22(1):197. 10.1186/s12931-021-01791-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee JH, Park HJ, Kim S, Kim YJ, Kim HC. Epidemiology and comorbidities in idiopathic pulmonary fibrosis: a nationwide cohort study. BMC Pulm Med. 2023;23(1):54. 10.1186/s12890-023-02340-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huh K, Kim YE, Ji W, Kim DW, Lee EJ, Kim JH, Kang JM, Jung J. Decrease in hospital admissions for respiratory diseases during the COVID-19 pandemic: a nationwide claims study. Thorax. 2021;76(9):939–41. 10.1136/thoraxjnl-2020-216526 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.