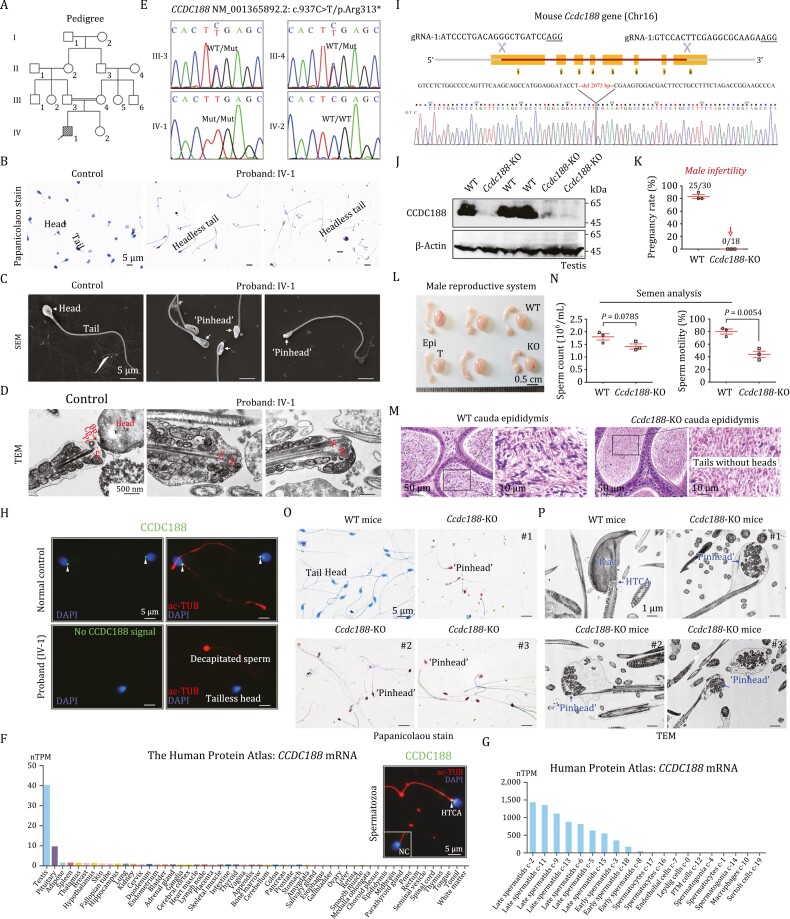
Figure 1.
Identification of a homozygous nonsense variant in the CCDC188 gene in an infertile patient with ASS and generation of Ccdc188-KO mice. (A) Pedigree of the recruited patient’s family. Black-filled square indicates the proband (IV-1). (B) Sperm morphology of the proband and a normal control was examined by Papanicolaou staining. Scale bars, 5 μm. (C) SEM was performed to reveal the ‘pinhead’ structure (arrows) in the front of the headless flagella in the proband. Scale bars, 5 μm. (D) TEM analysis indicated the ultrastructure of HTCA of sperm from the proband and a normal control. Bp, basal plate; Cp, capitulum; Pc, proximal centriole; Sc, segmented column. Scale bars, 500 nm. (E) Sanger sequencing validation of the variant of CCDC188, c.937C > T/p.Arg313*, in the proband and all available family members. (F) Among different human tissues, CCDC188 mRNA was predominantly expressed in the testes, according to the Human Protein Atlas. Immunofluorescence staining of CCDC188 and acetylated Tubulin (ac-TUB) in a human spermatozoon. Scale bar, 5 μm. NC, negative control, IgG was used. (G) In human testes, CCDC188 mRNA was restricted to early/late spermatids, according to the Human Protein Atlas. (H) Immunofluorescence staining of CCDC188 on spermatozoa from the proband and a normal control. Flagella and nucleus were stained with ac-TUB and DAPI, respectively. Arrowheads indicated CCDC188 signals. Scale bar, 5 μm. (I) Schematic illustration of the targeting strategy for generating Ccdc188-KO mice by using CRISPR/Cas9 technology. gRNA sequences and Sanger sequencing were provided. (J) Immunoblotting of CCDC188 was performed in the testis protein lysates of WT mice and Ccdc188-KO mice. β-Actin was used as an internal control. (K) Fertility assessment experiments were performed on three adult Ccdc188-KO male mice and three WT male littermates for 2 months. (L) Reproductive system of adult WT mice and Ccdc188-KO mice. T, testis; Epi, epididymis. Scale bars, 0.5 cm. (M) Histological morphology of cauda epididymis from WT mice and Ccdc188-KO mice by haematoxylin-eosin (H&E) staining. Scale bars, 50 or 10 µm. (N) Sperm counts were counted with a fertility counting chamber under a light microscope and total motility was assessed by a CASA system. Data are presented as the mean ± SD (n = 3 each group), Student’s t test. (O) Morphological analyses of sperm in WT mice and Ccdc188-KO mice by Papanicolaou staining. Arrows indicated the pinheads. Scale bars, 5 μm. (P) TEM images of sperm from WT mice and Ccdc188-KO mice. Scale bars, 1 μm.