Abstract
Background
Treatment persistence among patients with psoriatic arthritis (PsA) is essential for achieving optimal treatment outcomes. Guselkumab, a fully human interleukin-23p19-subunit inhibitor, was approved by the United States (US) Food and Drug Administration for the treatment of active PsA in July 2020, with a dosing regimen of 100 mg at week 0, week 4, then every 8 weeks. In the Phase 3 DISCOVER-1 and DISCOVER-2 studies of patients with active PsA, 94% of guselkumab-randomized patients completed treatment through 1 year and 90% did so through 2 years (DISCOVER-2). Real-world evidence is needed to compare treatment persistence while following US prescribing guidelines (i.e., on-label persistence) for guselkumab versus subcutaneous (SC) tumor necrosis factor inhibitors (TNFis).
Methods
Adults with PsA receiving guselkumab or their first SC TNFi (i.e., adalimumab, certolizumab pegol, etanercept, or golimumab) between 14 July 2020 and 31 March 2022 were identified in the IQVIA PharMetrics® Plus database (first claim defined the treatment start date [index date]). Baseline characteristics and biologic use (biologic-naïve/biologic-experienced) were assessed during the 12-month period preceding the index date. Baseline characteristics were balanced between cohorts using propensity-score weighting based on the standardized mortality ratio approach. The follow-up period spanned from the index date until the earlier of the end of continuous insurance eligibility or end of data availability. On-label persistence, defined as the absence of treatment discontinuation (based on a gap of 112 days for guselkumab or 56 days for SC TNFi) or any dose escalation/reduction during follow-up, was assessed in the weighted treatment cohorts using Kaplan-Meier (KM) curves. A Cox proportional hazards model, further adjusted for baseline biologic use, was used to compare on-label persistence between the weighted cohorts.
Results
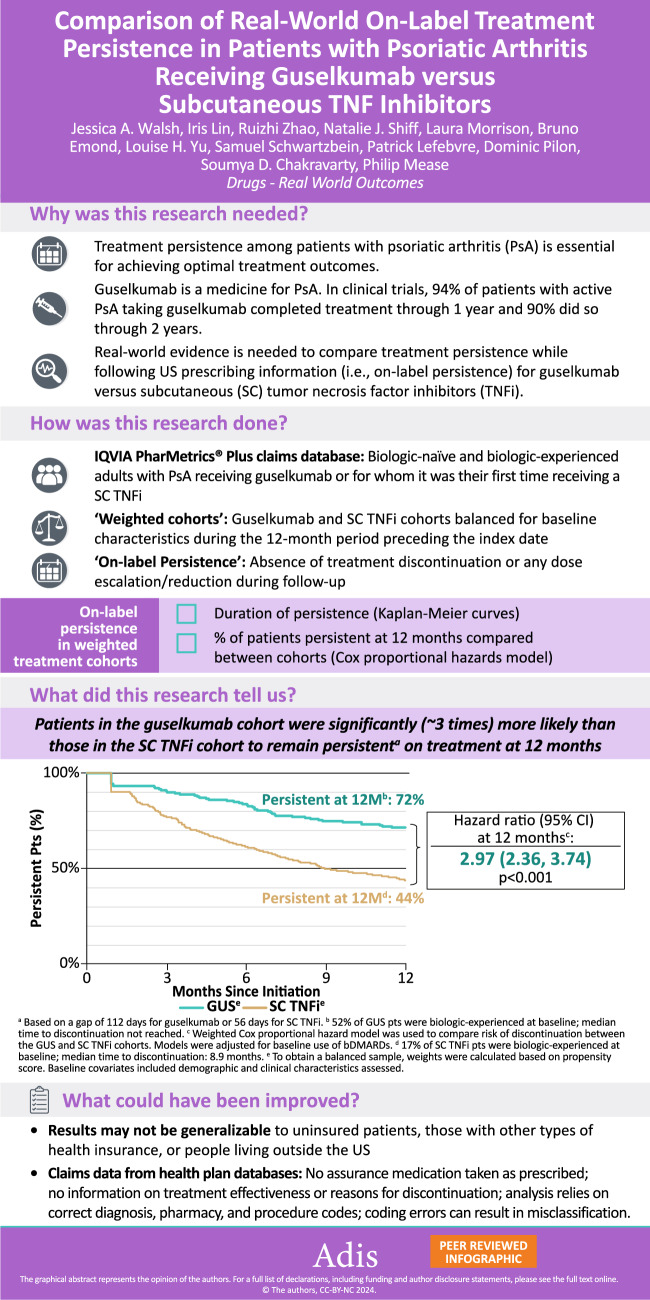
The guselkumab cohort included 526 patients (mean age 49.8 years; 61.2% female) and the SC TNFi cohort included 1953 patients (mean age: 48.5 years; 60.2% female). After weighting, baseline characteristics were well balanced with a mean follow-up of 12.3–12.4 months across cohorts; 51.5% of patients in the guselkumab cohort and 16.7% in the SC TNFi cohort received biologics in the 12-month baseline period. Respective rates of treatment persistence at 3, 6, 9, and 12 months were 91.2%, 84.1%, 75.9%, and 71.5% for the guselkumab cohort versus 77.3%, 61.6%, 50.0%, and 43.7% for the SC TNFi cohort (all log-rank p < 0.001). At 12 months, patients in the guselkumab cohort were 3.0 times more likely than patients in the SC TNFi cohort to remain persistent on treatment (p < 0.001). Median time to discontinuation was not reached for the guselkumab cohort and was 8.9 months for the SC TNFi cohort.
Conclusion
This real-world study employing US commercial health-plan claims data to assess on-label treatment persistence in PsA demonstrated that, at 12 months, guselkumab was associated with a 3 times greater likelihood of persistence compared with SC TNFi.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s40801-024-00428-z.
Key Points
| In July 2020, the United States (US) Food and Drug Administration approved guselkumab, a fully human interleukin-23p19-subunit inhibitor, for the treatment of active psoriatic arthritis (PsA) based on a dosing regimen of 100 mg at week 0, week 4, then every 8 weeks. |
| This retrospective study of patients with PsA in a US commercial health plan claims database, identified using a validated algorithm, compared on-label treatment persistence between those receiving guselkumab or their first subcutaneous (SC) tumor necrosis factor inhibitor (TNFi) during the study period. Persistence was defined as the absence of treatment discontinuation (based on a gap of 112 days for guselkumab or 56 days for SC TNFi) or any dose escalation/reduction during follow-up. |
| After applying propensity-score weighting based on the standardized mortality ratio approach, although more patients with PsA were biologic-experienced in the guselkumab (51.5%) versus SC TNFi (16.7%) cohort, patients receiving guselkumab were 3 times more likely than those receiving their first SC TNFi to be persistent on treatment through 1 year. Rates of persistence at 1 year were 71.5% and 43.7%, respectively, in the guselkumab and SC TNFi cohorts. |
Background
Psoriatic arthritis (PsA), a multidomain, systemic inflammatory disease occurring in ~30% of patients with psoriasis [1–4], is estimated to affect over 7.5 million adults in the United States (US) [5]. Manifestations include peripheral arthritis, skin and nail psoriasis, axial disease, enthesitis, and dactylitis [6]. Additionally, inflammatory bowel disease (IBD) and uveitis are considered PsA-related conditions, and obesity, cardiometabolic conditions, and mood disorders are commonly associated comorbidities [7]. PsA can result in a considerable burden on patients’ daily living and profoundly impact their health-related quality of life (HRQoL) [8–10].
Conventional pharmacological treatments for PsA include nonsteroidal anti-inflammatory drugs, glucocorticoids, and conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs), including methotrexate, which have shown modest efficacy in relieving certain PsA symptoms but are unable to delay radiographic progression [1, 2]. Biologic DMARDs (bDMARDs) indicated for PsA such as subcutaneous (SC) tumor necrosis factor inhibitors (TNFis) and interleukin (IL)-12/23, IL-17A, and IL-23 inhibitors have demonstrated clinical benefit across multiple disease domains [1, 2]. Following its approval in 2017 for the treatment of moderate-to-severe plaque psoriasis in adults, on 13 July 2020, guselkumab became the first and only fully human IL-23p19-subunit inhibitor to receive approval from the US Food and Drug Administration (FDA) for the treatment of active PsA [11]. The FDA-approved dosing regimen of 100 mg SC injection at Weeks 0, 4, and every 8 weeks thereafter for adults with active PsA was based on the results of the Phase 3, randomized, placebo-controlled DISCOVER-1 and DISCOVER-2 clinical trials that demonstrated the efficacy and favorable benefit-risk profile of guselkumab in this population [12, 13].
As patients with chronic conditions often require lifelong treatment, maintaining pharmacological treatment persistence for those with PsA is crucial for achieving optimal outcomes and improving HRQoL [8, 14–16]. Retrospective studies using real-world claims data show that persistence rates for SC and intravenous (IV) TNFi in patients with PsA newly initiated on biologics range from 51% to 56% at 12 months [14] and 18% to 22% at 24 months [17]. Due to its recent approval, real-world persistence data for guselkumab in patients with active PsA are limited. In the context of the DISCOVER-1 and DISCOVER-2 clinical trials, completion rates for patients randomized to guselkumab were 94% through 1 year and 90% through 2 years [18, 19].
Real-world data, such as those collected in an administrative health claims database, can provide evidence about treatment persistence in routine clinical care, which may differ from findings derived from stringently controlled clinical trials. This study utilized health plan claims data from a population of commercially insured patients with PsA in the US to compare persistence on treatment, based on the FDA-approved dosing regimen, between those receiving guselkumab and those receiving an initial SC TNFi.
Methods
Data Source
IQVIA PharMetrics® Plus [20] is a health plan claims database of predominantly commercially insured patients that contains fully adjudicated claims for inpatient and outpatient services, as well as outpatient prescription drugs, dates of service, demographic variables (e.g., age, gender, and geographic region), and start and stop dates of health plan enrollment. Information is available for more than 210 million unique enrollees across the US, with more than 95 million individuals having both medical and pharmacy benefits (40 million individuals in most recent years), and offers a diverse representation of geographic zones, employers, payers, healthcare providers, and therapeutic specialties.
IQVIA PharMetrics® Plus data are de-identified and comply with the Health Insurance Portability and Accountability Act (HIPAA) regulations. Therefore, no Institutional Review Board review was required. Data for this study were collected between 14 July 2019 and 30 September 2022 to allow for 12 months of data availability for the assessment of patient characteristics prior to guselkumab FDA approval (i.e., 13 July 2020) for the treatment of active PsA based on a dosing regimen of 100 mg at week 0, week 4, then every 8 weeks.
Study Design and Study Population
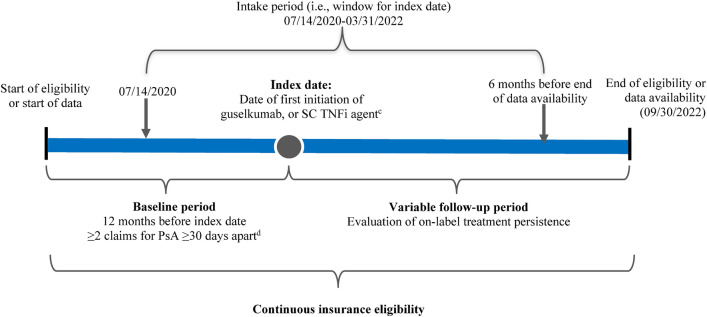
Adults with PsA who received an index biologic, i.e., guselkumab or first SC TNFi (adalimumab, certolizumab pegol, etanercept, or golimumab), between 14 July 2020 and 31 March 2022 (i.e., 6 months before the end of data availability) were included in the study (Fig. 1). The rationale for ending the intake period 6 months before the data cut-off was to allow patients in both cohorts to have sufficient follow-up time and an opportunity to discontinue the index medication. The date of the first claim for guselkumab or SC TNFi was defined as the index date, and baseline characteristics were assessed during the 12-month period of continuous insurance eligibility before the index date (i.e., baseline period). Based on a validated US claims-based algorithm for identifying PsA described by Lee and colleagues [21], patients were required to have ≥ 2 diagnoses for PsA (International Classification of Diseases, Tenth Revision [ICD-10] code: L40.5x) ≥ 30 days apart during the baseline period or on the index date as well as ≥ 1 prescription claim for guselkumab or an SC TNFi, the first of which defined the index date. For biologic agents to be covered by a commercial health plan, a patient must meet prior authorization criteria, which ensure that the medication is being prescribed for the intended use defined by FDA labeling [11]. Given that the index biologics evaluated as part of this study have been FDA-approved for the treatment of active PsA, a claim for the index biologic was considered a proxy for active disease.
Fig. 1.
Study design schema. a,bICD-10 International Classification of Disease, 10th revision, PsA psoriatic arthritis, SC subcutaneous, TNFi tumor necrosis factor inhibitor, US United States. aA validated algorithm for identifying patients with PsA in US claims data was used [21]. bPatients could be biologic-naïve or biologic-experienced during the 12-month baseline period but were naïve to treatment with guselkumab or TNFi agents. cThe SC TNFi cohort included patients receiving a first SC TNFi. dDiagnoses for PsA include claims on the index date. Patients with PsA were identified based on ≥ 2 PsA diagnoses (ICD-10 code L40.5x) ≥ 30 days apart and ≥ 1 prescription claim for a PsA-related medication
Patients were identified as either biologic-naïve or biologic-experienced based on prior bDMARD use (other than guselkumab, SC TNFi, or IV TNFi) during the 12-month baseline period. However, patients were excluded if they had received ≥ 1 claim for any of the drugs under study (i.e., guselkumab or SC TNFi) or IV TNFi (i.e., infliximab or golimumab) any time during the period of continuous eligibility before the index date, in order to compare patients receiving either guselkumab or their first SC TNFi rather than patients cycling among TNFi therapies due to inadequate response or intolerance. In addition, patients were excluded if they initiated more than one index biologic on the index date or had ≥ 1 diagnosis for other potentially confounding rheumatic diseases in the baseline period. The latter included ankylosing spondylitis (ICD-10 code: M45.x), other inflammatory arthritides (i.e., gout [ICD-10 codes: M10, M1A], calcium pyrophosphate dihydrate crystal deposition disease [ICD-10 codes: M11.20, M11.80], non-radiographic axial spondyloarthritis [ICD-10 code: M45.A], post-infectious and reactive arthritis [ICD-10 code: M02.x]), other spondyloarthropathies (ICD-10 code: M48), rheumatoid arthritis (ICD-10 codes: M05, M06, M08, M12), systemic connective tissue disorders (ICD-10 codes: M30–M35.x), relapsing polychondritis (ICD-10 code: M94.1), or unclassified connective tissue disease (ICD-10 code: L94.9). On-label persistence with the index agent was evaluated during the follow-up period, which spanned from the index date until the earlier of the end of continuous insurance eligibility or end of data availability.
Outcome Measures and Statistical Analyses
The outcome for this study was on-label persistence with the index biologic, defined as the absence of treatment discontinuation, and patients were censored at any dose escalation/reduction that was inconsistent with the respective FDA label dosing instructions for each agent. The primary definition of treatment discontinuation corresponded to a gap spanning twice the longest duration of time between administrations as per the FDA label [11, 22–25], incorporating the mode of days of supply observed in the data for each individual SC TNFi agent. Specifically, while adalimumab and certolizumab pegol dosing is indicated every 14 days, the mode of days of supply observed in the data was 28 days, as each claim typically contains two injections. Similarly, while etanercept is indicated for administration every 7 days, the mode of days of supply was 28 days owing to each claim typically comprising four injections. The longest dosing interval according to the FDA labels was selected for each agent considering that shorter dosing intervals are associated only with loading doses. As such, a 112-day gap (2 × 56 days) defined guselkumab discontinuation and a 56-day gap (2 × 28 days) defined SC TNFi discontinuation in the primary analyses. In sensitivity analyses, two additional definitions of discontinuation were assessed. In the first sensitivity analysis, the treatment gap was defined as the longest duration of time between administrations as per the FDA label (i.e., 56-day gap to define guselkumab discontinuation and 28-day gap to define SC TNFi discontinuation) [11, 22–25]. To evaluate persistence based on the same gap definition for all index biologics, the second sensitivity analysis employed a fixed 112-day gap to define discontinuation. If discontinuation was not observed, patients were censored at the earliest of the date of first observed off-label claim (i.e., the first observed dose escalation or reduction relative to the dosing instructions given by the respective FDA label, due to either a change in time between administrations or change in the number of injections per claim) or the last day of index agent supply preceding the end of the follow-up period.
Given the potential discrepancy between observed days of supply and the interval between claims in administrative databases, caused by restrictions on the maximum days of supply imposed by some health plans [26], the days of supply were conservatively imputed for both medical and pharmacy claims. For guselkumab medical claims, the days of supply were imputed as 28 days for the first claim and 56 days for all later claims, per the FDA label [11]. For guselkumab pharmacy claims, the days of supply were imputed as 28 days for the first claim with days of supply > 30 days, whereas second or later claim imputations were based on the time to next claim. For claims with missing days of supply or days of supply < 56 days, the days of supply were imputed as 28 days if the time to next claim was < 42 days, 56 days if the time to next claim was 42–70 days, and 84 days if the time to next claim was > 70 days. For SC TNFi medical claims, the days of supply were imputed as 28 days based on the mode of days of supply typically observed in pharmacy claims; no imputations were made for SC TNFi pharmacy claims, as the days of supply were largely consistent with respective FDA labels [22–25].
Baseline demographic and clinical characteristics were balanced between patients in the guselkumab and SC TNFi cohorts using propensity-score (PS) weighting calculated over the selection of treatment (i.e., guselkumab or an SC TNFi) based on the standardized mortality ratio (SMR) weighting approach. In this approach, each patient was attributed a weight based on the average treatment effect among the treated: 1 for patients in the guselkumab cohort and PS/(1-PS) for the SC TNFi cohort [27, 28]. Weights were then normalized using the mean weight so that the sample size of the weighted cohorts was the same as that of the unweighted cohorts. Covariates utilized in the SMR weighting included several baseline patient characteristics (see Online Supplemental Material [OSM] Methods). The balance of baseline demographic and clinical characteristics post-weighting was evaluated using standardized differences, whereby standardized differences < 10% indicated balance [29]. Due to the difference in prior bDMARD use during the 12-month baseline period in unweighted cohorts, this variable was not included in the SMR weighting as its inclusion meant there was not sufficient overlap in the PS distribution between treatment cohorts [30]. Utilizing the SMR-weighted treatment cohorts, Kaplan-Meier (KM) analysis was performed to assess the proportion of patients with on-label treatment persistence over time, up to 12 months following the index date. For example, in weighted cohorts, a patient with a weight of 1.5 who discontinues treatment would contribute an equivalent of 1.5 events. Cox proportional hazard models were used to compare on-label persistence between the SMR-weighted treatment cohorts at specific time points during follow-up, adjusting for prior bDMARD use in the 12-month baseline period, which was the only baseline demographic or clinical characteristic that remained imbalanced after weighting based on standardized differences > 10%.
Results
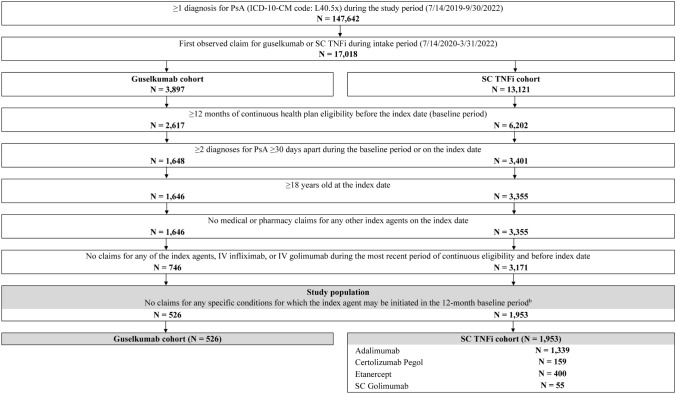
After applying the study’s selection criteria, the guselkumab cohort included 526 patients, among whom 48.5% were biologic-naïve and 51.5% were biologic-experienced during the 12-month baseline period, and the SC TNFi cohort included 1953 patients, among whom 87.9% were biologic-naïve and 12.1% were biologic-experienced (Fig. 2).
Fig. 2.
Identification of the study population of patients with PsA receiving guselkumab or an SC TNFi.aICD-10 International Classification of Disease, 10th revision, IV intravenous, PsA psoriatic arthritis, SC subcutaneous, TNFi tumor necrosis factor inhibitor. aThe SC TNFi cohort is defined as patients with an index claim for an SC TNFi (i.e., adalimumab, certolizumab pegol, etanercept, or golimumab). bSpecific conditions for which the index agent may be initiated included ankylosing spondylitis, calcium pyrophosphate deposition disease, gout, non-radiographic axial spondyloarthritis, other spondyloarthropathies, post infectious and reactive arthropathies, relapsing polychondritis, rheumatoid arthritis, systemic connective tissue disorders, or unclassified connective tissue disease
Baseline Characteristics
Baseline demographic and clinical characteristics for the unweighted and weighted cohorts are reported in Table 1. After implementation of weighting, baseline characteristics were well balanced between patients in the guselkumab and SC TNFi cohorts, except for prior bDMARD use during the baseline period (51.5% in the guselkumab cohort and 16.7% in the SC TNFi cohort; Table 1). After weighting, the mean age was 49.8 years in the guselkumab cohort and 49.2 years in the SC TNFi cohort, and 61.2% and 61.1% of the cohorts, respectively, were female. A psoriasis diagnosis was observed in 89.4% of patients in the guselkumab cohort and 88.0% of those in the SC TNFi cohort. The most common comorbidity was hyperlipidemia (38.8% in the guselkumab cohort and 36.1% in the SC TNFi cohort), and in the guselkumab and SC TNFi cohorts, respectively, 3.2% and 3.4% of patients had IBD, while 0.4% and 0.5% had uveitis. The use of csDMARDs during the baseline period was observed in 22.4% and 24.1% of patients in the guselkumab and SC TNFi cohorts, respectively; targeted synthetic DMARDs were used by 18.1% and 18.4%, respectively, of the patients in those cohorts. Among patients with biologic use during the 12-month baseline period, 84.1% of those in the guselkumab cohort and 92.4% of those in the SC TNFi cohort received one prior biologic, while 15.9% and 7.6%, respectively, received ≥ 2 prior biologics.
Table 1.
Demographic and clinical characteristics in the 12-month baseline period
| Mean ± SD [median] or n (%) | Unweighted cohorts | Weighted cohortsa | ||||
|---|---|---|---|---|---|---|
| Guselkumab | SC TNFi | Std. diff., % | Guselkumab | SC TNFi | Std. diff., % | |
| N = 526 | N = 1953 | N = 526 | N = 1953 | |||
| Age at index date (years) | 49.8 ± 11.7 [50.7] | 48.5 ± 11.4 [49.6] | 10.6 | 49.8 ± 11.7 [50.7] | 49.2 ± 11.6 [50.3] | 5.2 |
| Female | 322 (61.2) | 1176 (60.2) | 2.1 | 322 (61.2) | 1193 (61.1) | 0.3 |
| US region of residence at index date | ||||||
| South | 276 (52.5) | 864 (44.2) | 16.5 | 276 (52.5) | 1008 (51.6) | 1.7 |
| Midwest | 119 (22.6) | 460 (23.6) | 2.2 | 119 (22.6) | 443 (22.7) | 0.2 |
| Northeast | 78 (14.8) | 335 (17.2) | 6.3 | 78 (14.8) | 290 (14.9) | 0.1 |
| West | 53 (10.1) | 292 (15.0) | 14.8 | 53 (10.1) | 209 (10.7) | 2.0 |
| Unknown | 0 (0.0) | 2 (0.1) | 4.5 | 0 (0.0) | 3 (0.1) | 5.5 |
| Insurance type at index date | ||||||
| Preferred provider organization | 403 (76.6) | 1463 (74.9) | 4.0 | 403 (76.6) | 1501 (76.9) | 0.6 |
| Health maintenance organization | 65 (12.4) | 270 (13.8) | 4.4 | 65 (12.4) | 245 (12.6) | 0.6 |
| Otherb | 58 (11.0) | 220 (11.3) | 0.8 | 58 (11.0) | 207 (10.6) | 1.4 |
| Medicare Advantage enrollment | ||||||
| Not enrolled | 511 (97.1) | 1923 (98.5) | 9.0 | 511 (97.1) | 1910 (97.8) | 4.1 |
| Enrolled | 15 (2.9) | 30 (1.5) | 9.0 | 15 (2.9) | 43 (2.2) | 4.1 |
| Relationship of patient to the primary beneficiary at index date | ||||||
| Self | 306 (58.2) | 1169 (59.9) | 3.4 | 306 (58.2) | 1205 (61.7) | 7.2 |
| Spouse | 120 (22.8) | 409 (20.9) | 4.5 | 120 (22.8) | 405 (20.7) | 5.1 |
| Child | 5 (1.0) | 48 (2.5) | 11.7 | 5 (1.0) | 21 (1.1) | 1.2 |
| Unknown | 95 (18.1) | 327 (16.7) | 3.5 | 95 (18.1) | 322 (16.5) | 4.2 |
| Year of index date | ||||||
| 2020 | 89 (16.9) | 458 (23.5) | 16.3 | 89 (16.9) | 332 (17.0) | 0.3 |
| 2021 | 346 (65.8) | 1225 (62.7) | 6.4 | 346 (65.8) | 1284 (65.8) | 0.0 |
| 2022 | 91 (17.3) | 270 (13.8) | 9.6 | 91 (17.3) | 336 (17.2) | 0.2 |
| Time between latest observed PsA diagnosis to index date (months) | 1.4 ± 1.7 [0.8] | 0.9 ± 1.2 [0.6] | 31.0 | 1.4 ± 1.7 [0.8] | 1.2 ± 1.6 [0.7] | 9.6 |
| Baseline Quan-CCI | 0.6 ± 1.4 [0.0] | 0.5 ± 1.2 [0.0] | 7.2 | 0.6 ± 1.4 [0.0] | 0.6 ± 1.4 [0.0] | 0.6 |
| Prior conditions | ||||||
| Hyperlipidemia | 204 (38.8) | 616 (31.5) | 15.2 | 204 (38.8) | 706 (36.1) | 5.5 |
| Osteoarthritis | 130 (24.7) | 694 (35.5) | 23.7 | 130 (24.7) | 521 (26.7) | 4.4 |
| Diabetes | 94 (17.9) | 256 (13.1) | 13.2 | 94 (17.9) | 312 (16.0) | 5.1 |
| IBDc | 17 (3.2) | 85 (4.4) | 5.9 | 17 (3.2) | 67 (3.4) | 1.2 |
| Peripheral vascular disease | 6 (1.1) | 40 (2.0) | 7.2 | 6 (1.1) | 23 (1.2) | 0.2 |
| Uveitis | 2 (0.4) | 12 (0.6) | 3.3 | 2 (0.4) | 9 (0.5) | 1.5 |
| Psoriasisd | 470 (89.4) | 1348 (69.0) | 51.7 | 470 (89.4) | 1718 (88.0) | 4.4 |
| Smoking | 70 (13.3) | 192 (9.8) | 10.9 | 70 (13.3) | 237 (12.2) | 3.5 |
| Any prior PsA treatment | 385 (73.2) | 1255 (64.3) | 19.4 | 385 (73.2) | 1012 (51.8) | 45.3 |
| bDMARDse | 271 (51.5) | 236 (12.1) | 93.5 | 271 (51.5) | 327 (16.7) | 78.9 |
| 1 | 228 (84.1) | 218 (92.4) | 77.5 | 228 (84.1) | 302 (92.4) | 64.3 |
| ≥ 2 | 43 (15.9) | 18 (7.6) | 36.1 | 43 (15.9) | 25 (7.6) | 34.3 |
| csDMARDsf | 118 (22.4) | 894 (45.8) | 50.8 | 118 (22.4) | 471 (24.1) | 4.0 |
| tsDMARDsg | 95 (18.1) | 316 (16.2) | 5.0 | 95 (18.1) | 359 (18.4) | 0.8 |
| Prior non-narcotic analgesics use | 22 (4.2) | 48 (2.5) | 9.6 | 22 (4.2) | 65 (3.3) | 4.5 |
| Prior corticosteroids use | 375 (71.3) | 1294 (66.3) | 10.9 | 375 (71.3) | 1316 (67.4) | 8.5 |
| Prior opioids use | 182 (34.6) | 538 (27.5) | 15.3 | 182 (34.6) | 639 (32.7) | 4.0 |
bDMARD biologic disease-modifying anti-rheumatic drug, CCI Charlson comorbidity index, csDMARD conventional synthetic disease-modifying anti-rheumatic drug, CTLA cytotoxic T lymphocyte-associated antigen, IBD inflammatory bowel disease, ICD-10 International Classification of Disease, 10th revision, IL interleukin, IV intravenous, PsA psoriatic arthritis, SC subcutaneous, SD standard deviation, Std. Diff. standardized difference, TNFi tumor necrosis factor inhibitor, tsDMARD targeted synthetic disease-modifying anti-rheumatic drug, US United States. aPropensity score weighting based on the standardized mortality ratio weighting approach was used to adjust for differences in baseline characteristics between the guselkumab and SC TNFi cohorts. Weights were estimated using a multivariable logistic regression model. Baseline covariates included all demographic and clinical characteristics reported in this table with the exception of baseline use of bDMARDs, which was included in the adjusted Cox proportional hazard models. bPoint-of-service, consumer directed health care, indemnity/traditional, and unknown plan type. cUnclassified IBD, Crohn’s disease, and ulcerative colitis. dDefined based on ICD-10 code L40.x (excluding L40.5). eIL-17A inhibitors (i.e., secukinumab and ixekizumab), IL-12/23 inhibitor (i.e., ustekinumab), anti-CTLA-4 agent (i.e., abatacept), and IL-23p19-subunit inhibitor (i.e., risankizumab). The proportion of patients with 1 and ≥ 2 bDMARDs is reported among those with any bDMARD use. fMethotrexate, leflunomide, cyclosporine, mycophenolate, and azathioprine. gApremilast, deucravacitinib, and Janus kinase inhibitors (i.e., upadacitinib, baricitinib, and tofacitinib)
On-Label Persistence
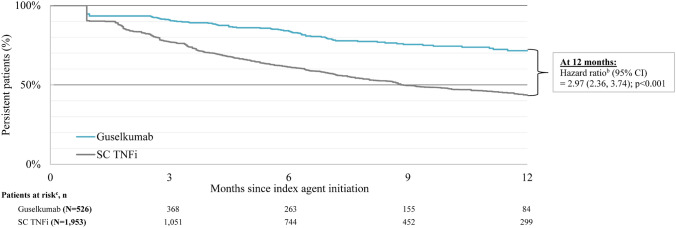
The mean follow-up times were 12.3 months in the guselkumab cohort and 12.4 months in the SC TNFi cohort. Using the primary definition of discontinuation, which reflects a gap of twice the duration of time between administrations per the FDA label, the KM rates of on-label persistence in weighted cohorts at 3, 6, 9, and 12 months were 91.2%, 84.1%, 75.9%, and 71.5%, respectively, for the guselkumab cohort and 77.3%, 61.6%, 50.0%, and 43.7%, respectively, for the SC TNFi cohort (all log-rank p < 0.001; Table 2). Relative to patients in the SC TNFi cohort, those in the guselkumab cohort were 3.0 times more likely to remain persistent on treatment at 12 months (95% confidence interval [CI] 2.4–3.7; p < 0.001; Fig. 3). The median time to discontinuation was not reached for the guselkumab cohort, and, in contrast, was 8.9 months for the SC TNFi cohort.
Table 2.
On-label persistence through 12 months in weighted guselkumab and SC TNFi cohorts: aprimary analysis (gap of twice the duration of time between administrations as per FDA label)
| Cox proportional hazards modelb | 3 months | 6 months | 9 months | 12 months |
|---|---|---|---|---|
| Patients at risk, n (%)c | ||||
| Guselkumab (N = 526) | 368 (70.0) | 263 (50.0) | 155 (29.5) | 84 (16.0) |
| SC TNFi (N = 1953) | 1051 (53.8) | 744 (38.1) | 452 (23.1) | 299 (15.3) |
| Hazard ratios (95% CI) | 3.41 (2.41; 4.80) | 3.30 (2.51; 4.33) | 3.06 (2.41; 3.88) | 2.97 (2.36; 3.74) |
| Chi-square p value | < 0.001* | < 0.001* | < 0.001* | < 0.001* |
| KM persistence, % (95% CI) | ||||
| Guselkumab | 91.2 (82.8; 95.6) | 84.1 (76.7; 89.4) | 75.9 (68.3; 81.9) | 71.5 (63.2; 78.3) |
| SC TNFi | 77.3 (73.1; 80.9) | 61.6 (56.8; 66.1) | 50.0 (44.4; 55.3) | 43.7 (37.3; 49.8) |
| Log-rank test p-value | < 0.001* | < 0.001* | < 0.001* | < 0.001* |
bDMARD biologic disease-modifying anti-rheumatic drug, CI confidence interval, FDA Food and Drug Administration, KM Kaplan-Meier, SC subcutaneous, TNFi tumor necrosis factor inhibitor. *Denotes statistical significance based on a threshold of p < 0.05. aPropensity score weighting based on the standardized mortality ratio weighting approach was used to adjust for differences in baseline characteristics between the guselkumab and SC TNFi cohorts. Weights were estimated using a multivariable logistic regression model. Baseline covariates included all demographic and clinical characteristics reported in Table 1, with the exception of baseline use of bDMARDs, which was included in the adjusted Cox proportional hazard models. bCox proportional hazard models were used to compare risk of discontinuation between the weighted guselkumab and SC TNFi cohorts. Models were adjusted for baseline use of bDMARDs. cPatients at risk of having the event are patients who have not had the event and have not been lost to follow-up at that point in time
Fig. 3.
Kaplan-Meier analysis of on-label persistence in weighted guselkumab and SC TNFi cohorts: aprimary analysis (gap of twice the duration of time between administrations as per FDA label). bDMARDbiologic disease-modifying anti-rheumatic drug, CIconfidence interval, FDA Food and Drug Administration, SC subcutaneous, TNFi tumor necrosis factor inhibitor. a Propensity score weighting based on the standardized mortality ratio weighting approach was used to adjust for differences in baseline characteristics between the guselkumab and SC TNFi cohorts. Weights were estimated using a multivariable logistic regression model. Baseline covariates included all demographic and clinical characteristics reported in Table 1, with the exception of baseline use of bDMARDs, which was included in the adjusted Cox proportional hazard models. b Cox proportional hazard models were used to compare risk of discontinuation between the weighted guselkumab and SC TNFi cohorts. Models were adjusted for baseline use of bDMARDs. c Patients at risk of having the event are patients who have not had the event and have not been lost to follow-up at that point in time
Consistent results were observed with each sensitivity analysis conducted. For the first sensitivity analysis, in which the discontinuation gap was defined as the longest duration between administrations according to the FDA label, the KM rates of on-label persistence in weighted cohorts at 3, 6, 9, and 12 months were 87.1%, 76.4%, 66.7%, and 59.2%, respectively, for the guselkumab cohort versus 72.3%, 55.0%, 41.5%, and 33.5%, respectively, for the SC TNFi cohort (all log-rank p < 0.001; OSM Table 1). At 12 months, patients in the guselkumab cohort were 2.4 times more likely to remain persistent on treatment than those in the SC TNFi cohort (95% CI: 2.0–2.9; p < 0.001; OSM Fig. 1). The median time to discontinuation was 18.4 months for the guselkumab cohort versus 6.9 months for the SC TNFi cohort. For the second sensitivity analysis, employing a fixed gap of 112 days, the KM rates of on-label persistence in weighted cohorts at 3, 6, 9, and 12 months were 91.2%, 84.1%, 75.9%, and 71.5%, respectively, for the guselkumab cohort versus 82.6%, 68.7%, 58.9%, and 52.1%, respectively, for the SC TNFi cohort (all log-rank p < 0.001; OSM Table 2). At 12 months, patients in the guselkumab cohort were 2.4 times more likely to remain persistent on treatment (95% CI: 1.9–3.0; p < 0.001; OSM Fig. 2). While not reached in the guselkumab cohort, the median time to discontinuation was 13.8 months for the SC TNFi cohort.
Discussion
This retrospective claims-based study represents the first comparative analysis of real-world on-label treatment persistence for guselkumab, a selective IL-23p19-subunit inhibitor, versus SC TNFi for the treatment of PsA in the US. Results showed that patients treated with guselkumab were significantly more likely (~3 times) than those treated with SC TNFi to remain persistent with treatment at 12 months.
Based on a therapy exposure gap of twice the duration of time between administrations, 72% of patients with PsA who received guselkumab were persistent on treatment after 12 months compared with only 44% of patients who received an SC TNFi. Furthermore, the median time to treatment discontinuation was 9 months in the SC TNFi cohort whereas more than half of the patients in the guselkumab cohort continued treatment beyond 12 months. The current results are consistent with findings from previous studies investigating the real-world drug survival of SC TNFi in patients with PsA, i.e., 12-month persistence rates of ~40% to 50% and estimated durations of persistence of ~8 months, using US Medicare and commercial insurance claims data across various definitions of the therapy exposure gap (i.e., 60- or 90-day fixed gap), patient clinical characteristics (e.g., biologic-naïve or biologic-experienced and with co-occurring rheumatic disease), and geographic regions [31–33]. Significantly higher rates of treatment persistence with guselkumab versus a first SC TNFi are also aligned with a series of recent studies describing persistence among biologic-naïve and biologic-experienced patients with psoriasis who initiated biologics in US and non-US clinical practice, whereby guselkumab demonstrated high persistence and showed higher rates of persistence and disease remission relative to other biologics, including TNFi, IL-17A inhibitors, and IL-12/23 inhibitors [34–36]. Notably, in a recent analysis that used the same insurance claims database to compare on-label treatment persistence among patients with PsA initiated on guselkumab or an SC IL-17A inhibitor, those who initiated guselkumab were ~2 times more likely to remain persistent at each time point up to 12 months compared with those who initiated SC IL-17A inhibitors (data presented at the Rheumatology Winter Clinical Symposium on 14–17 February 2024 in Maui, Hawaii).
For patients with PsA receiving biologics, long-term persistence on treatment can be an integral determinant of optimal outcomes [8, 14–16]. However, previous studies have documented a general pattern of poor persistence on biologics among this population in clinical practice [31, 32, 37]. Within 2 years of treatment initiation, one study showed that the overall discontinuation rate was ~80% among biologic-naïve patients with PsA treated with injectable biologics, including SC TNFi [17]. In a Multinational Assessment of Psoriasis and Psoriatic Arthritis patient survey, 59% of patients with PsA were not receiving any therapy or were receiving only a topical therapy, despite 50% to 75% of patients self-reporting severe symptoms [38].
As a comprehensive measure, treatment persistence reflects many factors including treatment effectiveness and safety, disease severity and activity, the presence of comorbidities, as well as patient and physician preferences and awareness of biologics [14, 16, 37, 39]. Indeed, studies of patients with PsA treated with biologics, including TNFi, reported that inadequate symptom control and adverse effects were the most common predictors of switching or discontinuing treatments [38, 40, 41]. Recent US registry data from a largely treatment-refractory population of patients with active PsA show that after 6 months of persistent treatment with guselkumab, patients experienced significant improvements in peripheral joint and skin disease and patient-reported pain, with nearly 80% of patients maintaining on-label persistence with guselkumab at 6 months, which is consistent with the findings from the current study [42]. Though further analysis using long-term data is warranted, the high real-world persistence on guselkumab observed in this study aligns with the high patient retention rates of 94% through 1 year and 90% through 2 years in Phase 3 trials [18, 19].
Several methodological considerations strengthen the results reported in this study. The study sample comprised commercially insured patients whose baseline demographic and clinical characteristics were balanced between the guselkumab and SC TNFi cohorts using SMR weighting and for whom payer prior authorization requires that medications be used in accordance with FDA prescribing information. The use of a case-finding algorithm for PsA validated in US claims data, coupled with the prior authorization processes, provides confidence that this analysis included patients with active PsA, despite the lack of clinical measures of disease activity in this type of data source. The imputation of days of supply for certain agents was conducted based on the FDA label recommendations using the time to next claim as a proxy for days of supply, a common technique based on a previously published algorithm [26]. Although misclassification of off-label status for some patients may have occurred, more conservative estimates of the therapy exposure gap were employed in sensitivity analyses, the results of which were consistent with findings of the primary analysis. Specifically, significantly higher rates of guselkumab than SC TNFi persistence were also observed when exposure gaps to define treatment discontinuation were either the longest duration between administrations according to the FDA label or a fixed gap of 112 days. As such the potential misclassification resulting from the imputation of days of supply is expected to be minimal.
Limitations
In addition to the strengths highlighted above, the findings from this study should be interpreted in the context of some limitations, most of which are inherent to the use of health plan claims databases. As analyses depend on correct diagnosis, procedure, and drug codes, coding inaccuracies may have led to case misidentification. Claims data do not necessarily ensure that the medication was taken as prescribed, and neither evaluation of treatment effectiveness, reasons for discontinuation, nor date of death were available in the database. Relatedly, given the use of claims data and a 12-month baseline period, bDMARD exposure prior to the start of the baseline period was unknown. Therefore, misclassification could have occurred, but this is expected to be minimal as only an additional 3% of patients would have been reclassified as bio-experienced by extending the period for evaluating prior bDMARD use from the 12-month baseline period to the entire period of continuous insurance eligibility prior to the index date. In addition, certain clinical (e.g., patient- or clinician-reported outcomes of disease severity or relative burden of psoriasis vs. arthritis) or patient (e.g., race, ethnicity) characteristics that might influence outcomes were not available or may have been under-reported in the database, which may have resulted in residual confounding. Finally, results may not be generalizable to uninsured patients with PsA, those with other types of health insurance, or patients with PsA outside of the US.
Conclusions
This study was the first to use predominantly commercial health plan claims data to compare real-world persistence of guselkumab and SC TNFi per US FDA-approved labels among patients with PsA in the US. Results showed that over a 12-month period, guselkumab was associated with a 3 times greater likelihood of persistence than an initial SC TNFi. These findings are consistent with previous real-world studies reporting high persistence for patients treated with guselkumab, and support the high patient retention rates observed in clinical trials. Given the complexity and chronicity of PsA as a heterogenous disease, and the benefit of maintaining long-term disease control on optimizing clinical outcomes and HRQoL, long-term treatment persistence should be considered as part of the shared decision making in treatment selection.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Medical writing support was provided by professional medical writer, Loraine Georgy, PhD, MWC, an employee of Analysis Group, Inc., under the direction of the authors in accordance with Good Publication Practice guidelines (Ann Intern Med 2022;175: 1298-1304) and was funded by Janssen Scientific Affairs, LLC, a Johnson & Johnson company.
Declarations
Funding
This study was funded by Janssen Scientific Affairs, LLC, a Johnson & Johnson company. Authors who are employees of the study sponsor were involved in several aspects of the research, including designing the study. All authors were involved in interpretation of data and drafting or revising the writing of the manuscript.
Conflict of Interest
IL, NS, and SDC are employees of Janssen Scientific Affairs, LLC, a Johnson & Johnson company and stockholders of Johnson & Johnson. NS received salary support from the Childhood Arthritis and Rheumatology Research Alliance and owns or has owned stock in AbbVie, Gilead, Iovance, Jazz Pharmaceuticals, Novavax, and Viatris. RZ was an employee of Janssen Scientific Affairs, LLC, a Johnson & Johnson company at the time of study conduct. PL, DP, BE, LM, LHY, and SS are employees of Analysis Group, Inc., a consulting company that has received research funding from Janssen Scientific Affairs, LLC, a Johnson & Johnson company. JAW received research funding from Pfizer, Merck, AbbVie and served as a consultant for AbbVie, Janssen, Eli Lilly, Novartis, UCB. PM received research funding from AbbVie, Acelyrin, Amgen, Bristol Myers Squibb, Eli Lilly, Janssen, Novartis, Pfizer, Sun Pharma, and UCB, served as a consultant for AbbVie, Acelyrin, Aclaris, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Galapagos, Gilead, GlaxoSmithKline, Inmagene, Janssen, Novartis, Pfizer, Sun Pharma, UCB, and Ventyx, and received speaker fees from AbbVie, Amgen, Eli Lilly, Janssen, Novartis, Pfizer, and UCB.
Author Contributions
JAW contributed to the conceptualization, investigation, methodology, supervision, validation, visualization, writing of the original draft, and its review and editing. IL contributed to the conceptualization, funding acquisition, investigation, methodology, project administration, resources, supervision, validation, visualization, writing of the original draft, and its review and editing. RZ contributed to the conceptualization, funding acquisition, investigation, methodology, project administration, resources, supervision, validation, visualization, writing of the original draft, and its review and editing. NJS contributed to the conceptualization, funding acquisition, investigation, methodology, project administration, resources, supervision, validation, visualization, writing of the original draft, and its review and editing. LM contributed to the conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, software, supervision, validation, visualization, writing of the original draft, and its review and editing. BE contributed to the conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, software, supervision, validation, visualization, writing of the original draft, and its review and editing. LHY contributed to the conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, software, supervision, validation, visualization, writing of the original draft, and its review and editing. SS contributed to the conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, software, supervision, validation, visualization, writing of the original draft, and its review and editing. PL contributed to the conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, software, supervision, validation, visualization, writing of the original draft, and its review and editing. DP contributed to the conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, software, supervision, validation, visualization, writing of the original draft, and its review and editing. SDC contributed to the conceptualization, funding acquisition, investigation, methodology, project administration, resources, supervision, validation, visualization, writing of the original draft, and its review and editing. PM contributed to the conceptualization, investigation, methodology, supervision, validation, visualization, writing of the original draft, and its review and editing. All authors have reviewed and approved the final submitted manuscript and agree to be accountable for all aspects of the work.
Data Availability
The data that support the findings of this study are available from IQVIA PharMetrics® Plus. Restrictions apply to the availability of these data, which were used under license for this study.
Ethics Statement
Data were de-identified and comply with the patient requirements of the Health Insurance Portability and Accountability Act (HIPAA) of 1996; therefore, no review by an Institutional Review Board was required per Title 45 of CFR, Part 46.101(b)(4) (https://www.hhs.gov/ohrp/regulations-and-policy/regulations/45-cfr-46/#46.101).
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Code Availability
All analyses were conducted using SAS Enterprise Guide 7.1 (SAS Institute, Cary, NC, US). The SAS programs are proprietary materials of Analysis Group, Inc.; therefore, restrictions apply to the access of these codes, which cannot be made available publicly.
References
- 1.FitzGerald O, Ogdie A, Chandran V, Coates LC, Kavanaugh A, Tillett W, Leung YY, deWit M, Scher JU, Mease PJ. Psoriatic arthritis. Nat Rev Dis Primers. 2021;7:59. 10.1038/s41572-021-00293-y [DOI] [PubMed] [Google Scholar]
- 2.Ocampo DV, Gladman D. Psoriatic arthritis. F1000Res. 2019;8:1665. 10.12688/f1000research.19144.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alinaghi F, Calov M, Kristensen LE, Gladman DD, Coates LC, Jullien D, Gottlieb AB, Gisondi P, Wu JJ, Thyssen JP, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2019;80:251-65.e19. 10.1016/j.jaad.2018.06.027 [DOI] [PubMed] [Google Scholar]
- 4.Mease PJ, Gladman DD, Papp KA, Khraishi MM, Thaçi D, Behrens F, Northington R, Fuiman J, Bananis E, Boggs R. Prevalence of rheumatologist-diagnosed psoriatic arthritis in patients with psoriasis in European/North American dermatology clinics. J Am Acad Dermatol. 2013;69:729–35. 10.1016/j.jaad.2013.07.023 [DOI] [PubMed] [Google Scholar]
- 5.Armstrong AW, Mehta MD, Schupp CW, Gondo GC, Bell SJ, Griffiths CEM. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157:940–6. 10.1001/jamadermatol.2021.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gladman DD, Antoni C, Mease P, Clegg DO, Nash P. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis. 2005;64(Suppl 2):ii14–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coates LC, Soriano ER, Corp N, Bertheussen H, Callis Duffin K, Campanholo CB, Chau J, Eder L, Fernandez-Avila DG, FitzGerald O, et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA): updated treatment recommendations for psoriatic arthritis 2021. Nat Rev Rheumatol. 2022;18:465–79. 10.1038/s41584-022-00798-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gudu T, Gossec L. Quality of life in psoriatic arthritis. Expert Rev Clin Immunol. 2018;14:405–17. 10.1080/1744666X.2018.1468252 [DOI] [PubMed] [Google Scholar]
- 9.Coates LC, Orbai AM, Azevedo VF, Cappelleri JC, Steinberg K, Lippe R, Lim I, Eder L, Richette P, Weng MY, et al. Results of a global, patient-based survey assessing the impact of psoriatic arthritis discussed in the context of the Psoriatic Arthritis Impact of Disease (PsAID) questionnaire. Health Qual Life Outcomes. 2020;18:173. 10.1186/s12955-020-01422-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor WJ. Impact of psoriatic arthritis on the patient: through the lens of the WHO International Classification of Functioning, Health, and Disability. Curr Rheumatol Rep. 2012;14:369–74. 10.1007/s11926-012-0263-5 [DOI] [PubMed] [Google Scholar]
- 11.TREMFYA: Package insert. Horsham: Janssen Biotech, Inc, 2023.
- 12.Deodhar A, Helliwell PS, Boehncke WH, Kollmeier AP, Hsia EC, Subramanian RA, Xu XL, Sheng S, Agarwal P, Zhou B, et al. Guselkumab in patients with active psoriatic arthritis who were biologic-naive or had previously received TNFα inhibitor treatment (DISCOVER-1): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395:1115–25. 10.1016/S0140-6736(20)30265-8 [DOI] [PubMed] [Google Scholar]
- 13.Mease PJ, Rahman P, Gottlieb AB, Kollmeier AP, Hsia EC, Xu XL, Sheng S, Agarwal P, Zhou B, Zhuang Y, et al. Guselkumab in biologic-naive patients with active psoriatic arthritis (DISCOVER-2): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395:1126–36. 10.1016/S0140-6736(20)30263-4 [DOI] [PubMed] [Google Scholar]
- 14.Murage MJ, Tongbram V, Feldman SR, Malatestinic WN, Larmore CJ, Muram TM, Burge RT, Bay C, Johnson N, Clifford S, et al. Medication adherence and persistence in patients with rheumatoid arthritis, psoriasis, and psoriatic arthritis: a systematic literature review. Patient Prefer Adherence. 2018;12:1483–503. 10.2147/PPA.S167508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gottlieb A, Gratacos J, Dikranian A, van Tubergen A, Fallon L, Emir B, Aikman L, Smith T, Chen L. Treatment patterns, unmet need, and impact on patient-reported outcomes of psoriatic arthritis in the United States and Europe. Rheumatol Int. 2019;39:121–30. 10.1007/s00296-018-4195-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Helliwell P, Coates L, Chandran V, Gladman D, de Wit M, FitzGerald O, Kavanaugh A, Strand V, Mease PJ, Boehncke WH, et al. Qualifying unmet needs and improving standards of care in psoriatic arthritis. Arthritis Care Res (Hoboken). 2014;66:1759–66. 10.1002/acr.22404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walsh JA, Cai Q, Lin I, Fitzgerald T, Pericone CD, Chakravarty SD. Real-world 2-year treatment patterns among patients with psoriatic arthritis treated with injectable biologic therapies. Curr Med Res Opin. 2020;36:1245–52. 10.1080/03007995.2020.1754186 [DOI] [PubMed] [Google Scholar]
- 18.Ritchlin CT, Helliwell PS, Boehncke WH, Soriano ER, Hsia EC, Kollmeier AP, Chakravarty SD, Zazzetti F, Subramanian RA, Xu XL, et al. Guselkumab, an inhibitor of the IL-23p19 subunit, provides sustained improvement in signs and symptoms of active psoriatic arthritis: 1 year results of a phase III randomised study of patients who were biologic-naive or TNFα inhibitor-experienced. RMD Open. 2021;7:e001457. 10.1136/rmdopen-2020-001457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McInnes IB, Rahman P, Gottlieb AB, Hsia EC, Kollmeier AP, Xu XL, Jiang Y, Sheng S, Shawi M, Chakravarty SD, et al. Long-term efficacy and safety of guselkumab, a monoclonal antibody specific to the p19 subunit of interleukin-23, through two years: results from a phase III, randomized, double-blind, placebo-controlled study conducted in biologic-naive patients with active psoriatic arthritis. Arthritis Rheumatol. 2022;74:475–85. 10.1002/art.42010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.IQVIA. IQVIA Pharmetrics® Plus Database. IQVIA Inc; 2022. [Google Scholar]
- 21.Lee H, Ford JA, Jin Y, Cho SK, Santiago Ortiz AJ, Tong AY, Kim SC. Validation of claims-based algorithms for psoriatic arthritis. Pharmacoepidemiol Drug Saf. 2020;29:404–8. 10.1002/pds.4950 [DOI] [PubMed] [Google Scholar]
- 22.HUMIRA: Packaging insert. Abbott Laboratories. 2024.
- 23.CIMZIA: Packaging insert. UCB Inc. 2022.
- 24.ENBREL: Packaging insert. Immunex. 2021.
- 25.SIMPONI: Packaging insert. Horsham: Janssen Biotech, Inc. 2019.
- 26.Xu C, Ferrante SA, Fitzgerald T, Pericone CD, Wu B. Inconsistencies in the days supply values reported in pharmacy claims databases for biologics with long maintenance intervals. J Manag Care Spec Pharm. 2023;29:90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Austin PC. Variance estimation when using inverse probability of treatment weighting (IPTW) with survival analysis. Stat Med. 2016;35:5642–55. 10.1002/sim.7084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brookhart MA, Wyss R, Layton JB, Stürmer T. Propensity score methods for confounding control in nonexperimental research. Circ Cardiovasc Qual Outcomes. 2013;6:604–11. 10.1161/CIRCOUTCOMES.113.000359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Austin PC. Goodness-of-fit diagnostics for the propensity score model when estimating treatment effects using covariate adjustment with the propensity score. Pharmacoepidemiol Drug Saf. 2008;17:1202–17. 10.1002/pds.1673 [DOI] [PubMed] [Google Scholar]
- 30.Walker AM, Patrick AR, Lauer MS, Hornbrook MC, Marin MG, Platt R, Roger VL, Stang P, Schneeweiss S. A tool for assessing the feasibility of comparative effectiveness research. J Comp Eff Res. 2013;3:11–20. [Google Scholar]
- 31.Walsh JA, Adejoro O, Chastek B, Palmer JB, Hur P. Treatment patterns among patients with psoriatic arthritis treated with a biologic in the United States: descriptive analyses from an administrative claims database. J Manag Care Spec Pharm. 2018;24:623–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oelke KR, Chambenoit O, Majjhoo AQ, Gray S, Higgins K, Hur P. Persistence and adherence of biologics in US patients with psoriatic arthritis: analyses from a claims database. J Comp Eff Res. 2019;8:607–21. 10.2217/cer-2019-0023 [DOI] [PubMed] [Google Scholar]
- 33.Chastek B, Fox KM, Watson C, Gandra SR. Etanercept and adalimumab treatment patterns in psoriatic arthritis patients enrolled in a commercial health plan. Adv Ther. 2012;29:691–7. 10.1007/s12325-012-0039-3 [DOI] [PubMed] [Google Scholar]
- 34.Yiu ZZN, Becher G, Kirby B, Laws P, Reynolds NJ, Smith CH, Warren RB, Griffiths CEM, Group BS. Drug survival associated with effectiveness and safety of treatment with guselkumab, ixekizumab, secukinumab, ustekinumab, and adalimumab in patients with psoriasis. JAMA Dermatol. 2022;158:1131–41. 10.1001/jamadermatol.2022.2909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Egeberg A, Roseno NAL, Aagaard D, Lorup EH, Nielsen ML, Nymand L, Kristensen LE, Thyssen JP, Thomsen SF, Cordtz RL, et al. Drug survival of biologics and novel immunomodulators for rheumatoid arthritis, axial spondyloarthritis, psoriatic arthritis, and psoriasis—a nationwide cohort study from the DANBIO and DERMBIO registries. Semin Arthritis Rheum. 2022;53: 151979. 10.1016/j.semarthrit.2022.151979 [DOI] [PubMed] [Google Scholar]
- 36.Fitzgerald T, Zhdanava M, Pilon D, Shah A, Hilts A, Lefebvre P, Feldman SR. Long-term psoriasis control with guselkumab, adalimumab, secukinumab, or ixekizumab in the USA. Dermatol Ther (Heidelb). 2023;13:1053–68. 10.1007/s13555-023-00910-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haddad A, Gazitt T, Feldhamer I, Feld J, Cohen AD, Lavi I, Tatour F, Bergman I, Zisman D. Treatment persistence of biologics among patients with psoriatic arthritis. Arthritis Res Ther. 2021;23:44. 10.1186/s13075-021-02417-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lebwohl MG, Bachelez H, Barker J, Girolomoni G, Kavanaugh A, Langley RG, Paul CF, Puig L, Reich K, van de Kerkhof PC. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70(871–81):e1-30. [DOI] [PubMed] [Google Scholar]
- 39.Geale K, Lindberg I, Paulsson EC, Wennerström ECM, Tjärnlund A, Noel W, Enkusson D, Theander E. Persistence of biologic treatments in psoriatic arthritis: a population-based study in Sweden. Rheumatol Adv Pract. 2020;4:rkaa070. 10.1093/rap/rkaa070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Glintborg B, Ostergaard M, Krogh NS, Andersen MD, Tarp U, Loft AG, Lindegaard HM, Holland-Fischer M, Nordin H, Jensen DV, et al. Clinical response, drug survival, and predictors thereof among 548 patients with psoriatic arthritis who switched tumor necrosis factor alpha inhibitor therapy: results from the Danish Nationwide DANBIO Registry. Arthritis Rheumatol. 2013;65:1213–23. 10.1002/art.37876 [DOI] [PubMed] [Google Scholar]
- 41.Mease PJ, O’Brien J, Middaugh N, Kricorian G, Stryker S, Collier DH, Ogdie A. Real-world evidence assessing psoriatic arthritis by disease domain: an evaluation of the CorEvitas Psoriatic Arthritis/Spondyloarthritis Registry. ACR Open Rheumatol. 2023;5:388–98. 10.1002/acr2.11556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mease PJ, Ogdie A, Tesser J, Shiff NJ, Lin I, Chakravarty SD, Kelleman M, Dodge R, McLean RR, Broadwell A. Six-month persistence and multi-domain effectiveness of guselkumab in adults with psoriatic arthritis: real-world data from the CorEvitas Psoriatic Arthritis/Spondyloarthritis Registry. Rheumatol Ther. 2023;10:1–23. 10.1007/s40744-023-00582-w [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from IQVIA PharMetrics® Plus. Restrictions apply to the availability of these data, which were used under license for this study.