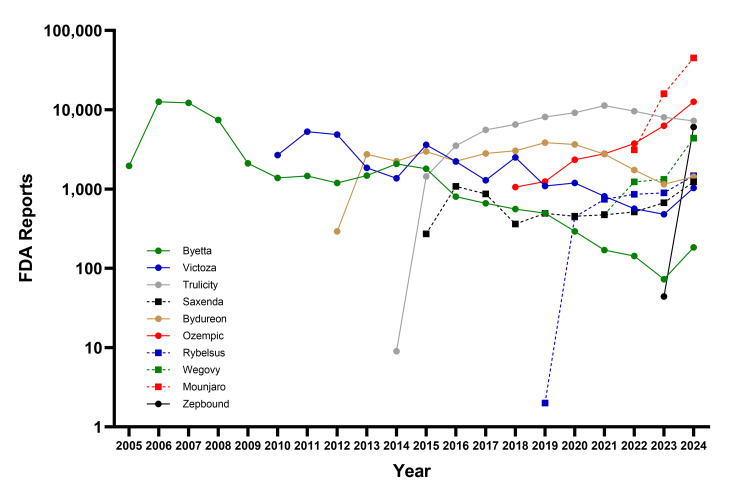
Figure 1. Annual trends in AE reports for GLP-1RAs in the FAERS database (2005-2024).
Data are reported through March 31, 2024. For the year 2024, the data have been annualized to represent the full year based on the information available.
AE, adverse event; FAERS, FDA Adverse Event Reporting System; GLP-1RA, glucagon-like peptide-1 receptor agonist