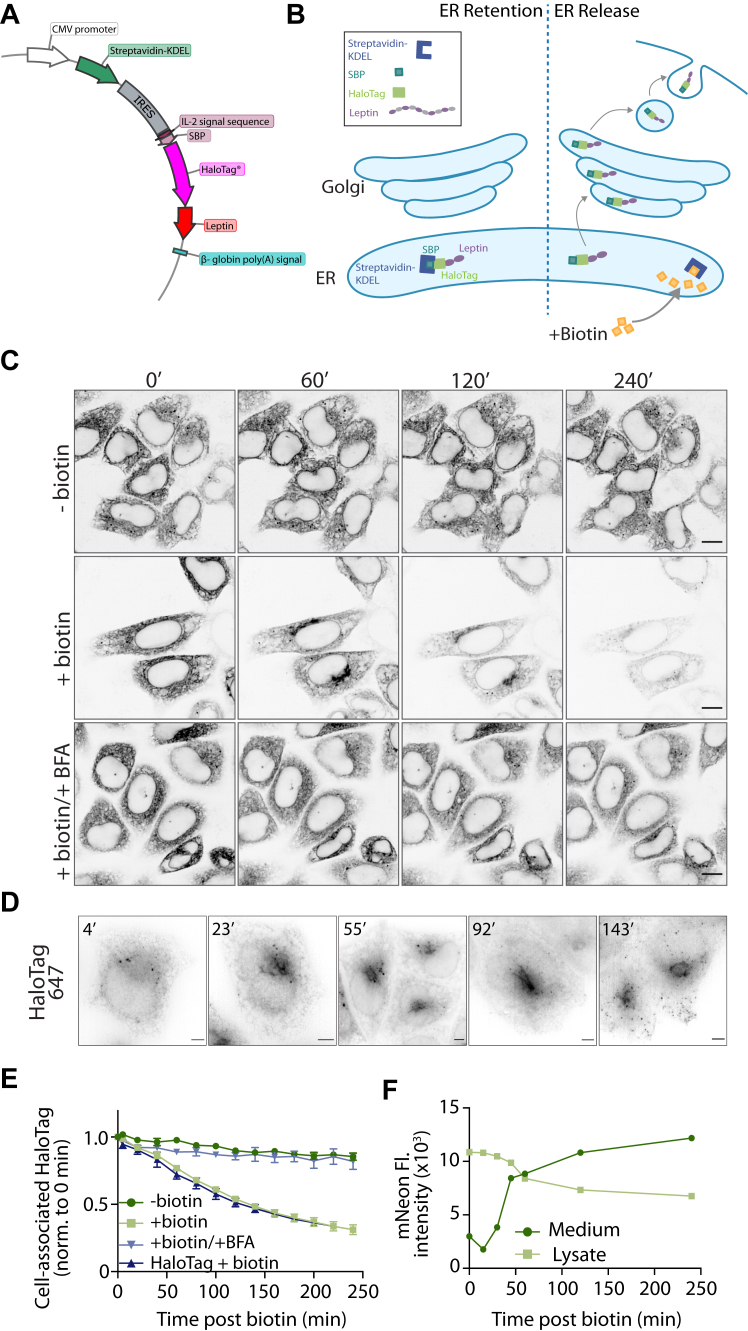
Figure 1.
Development and validation of a RUSH-based kinetic secretory assay to study leptin secretion. A, plasmid map of the piggybac vector used for stable cell line generation. A bicistronic internal ribosome entry site (IRES) vector is driven by the CMV promoter expressing an upstream streptavidin-KDEL and downstream a fusion protein of the signal peptide from IL-2, a HaloTag, and leptin. B, schematic of the RUSH-based system to monitor leptin secretion. Prior to biotin treatment, SBP-HaloTag-leptin is retained in the ER through an interaction between the SBP and the Strep-KDEL. Upon biotin addition, biotin outcompetes the SBP interaction and releases SBP-HaloTag-leptin from the ER, through the Golgi for secretion. C, live cell imaging of SBP-HaloTag-leptin in HeLa cells labeled with JFX646 Halo dye. Cells were treated with biotin and BFA where indicated and imaged at 0, 60, 120, and 240 min after biotin addition. Representative of n = 2; scale bar: 10 μm. D, live cell super-resolution imaging of SBP-HaloTag-leptin in HeLa cells labeled with JFX646 Halo dye. Images are from specified time points following biotin addition. Representative of n = 3; scale bar: 5 μm. E, kinetic analysis of SBP-HaloTag-leptin secretion using live cell imaging. Cells were treated with biotin and/or BFA where indicated. SBP-HaloTag was included as a control in this experiment (ctrl: dark blue). The fluorescence intensity of the cell-associated HaloTag signal was measured and normalized to the 0 min time point. n = 3 for SBP-Leptin-HaloTag cells ± biotin; n = 2 for SBP-Leptin-HaloTag cells + biotin/BFA and SBP-HaloTag cells (ctrl). Error bars = SEM. F, SBP-mNeonGreen-leptin HeLa cells were treated with biotin for specified time periods and the mNeonGreen fluorescence intensity in culture media (dark green) and cell lysate (light green) measured. n = 1.