Abstract
ABSTRACT
Background
Oesophageal squamous cell carcinoma (OSCC) poses a considerable health burden, particularly in regions such as East Asia. This study aims to investigate the long-term outcomes of OSCC patients who are smokers and drinkers.
Materials and methods
In this retrospective analysis, data from Sichuan Cancer Hospital and Institute Esophageal Cancer Case Management Database between January 2010 and December 2017 were examined. Patients were categorised into different groups based on their smoking and alcohol consumption history: None, Smoker, Non-Smoker, Smoke-Only, Drinker, Non-Drinker, Drinker-Only, and Both. Survival outcomes were compared between the groups using Kaplan-Meier analysis and propensity score matching (PSM). The primary outcome was overall survival (OS), measured from surgery to death or last follow-up in April 2022.
Results
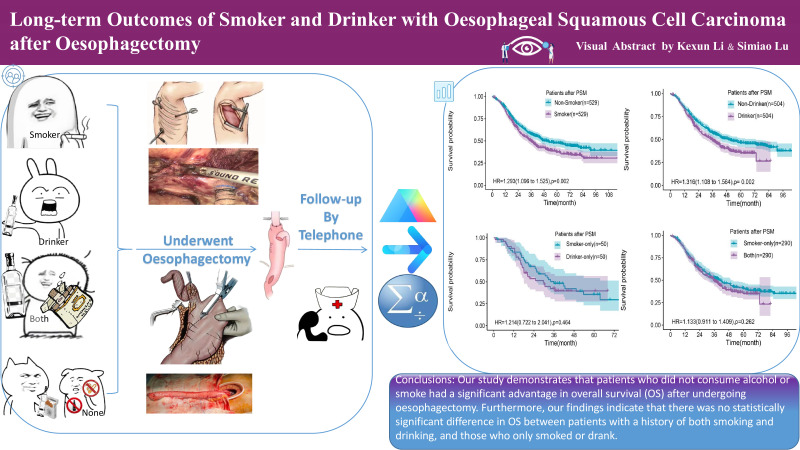
The OS median was 45.4 months for all patients after oesophagectomy. Smokers had a significantly lower median OS of 36.6 months compared with Non-Smokers with 66.2 months (p<0.001). Similarly, Drinkers had a lower median OS of 34.4 months compared with Non-Drinkers with 52.0 months (p<0.001). PSM analysis confirmed the significant differences in OS between Smokers and Non-Smokers (p=0.002) and between Drinkers and Non-Drinkers (p=0.002). Subgroup analyses showed no significant differences in OS between Group Another and Group Both, Group Smoker-Only and Group Drinker-Only, and Group Drinker-Only and Group Both. (figure 4)
Conclusion
Smoking and drinking were associated with significantly reduced OS in patients. However, no significant differences were found between the subgroups of patients who only smoked, only drank, or engaged in both habits.
Keywords: GASTROINTESTINAL SURGERY, SURGICAL ONCOLOGY, GASTRIC CANCER
WHAT IS ALREADY KNOWN ON THIS TOPIC
Oesophageal squamous cell carcinoma (OSCC) poses a significant health burden, particularly in East Asia. Smoking and alcohol consumption are well-established risk factors for OSCC. Previous studies have suggested that smoking and alcohol consumption negatively impact the prognosis of OSCC patients, but their relative impacts have not been directly compared.
WHAT THIS STUDY ADDS
This large retrospective study directly compared the long-term overall survival (OS) outcomes of OSCC patients based on their smoking and alcohol consumption history. The results clearly demonstrate that both smoking and drinking are associated with significantly reduced OS in OSCC patients, even after adjusting for other clinical and pathological factors. Importantly, the study found no significant differences in OS between patients who only smoked, only drank, or engaged in both habits, suggesting the maximum impact of these lifestyle factors may already be reached with just one habit. The study provides a comprehensive analysis of the prognostic impact of smoking and drinking in OSCC, using both Kaplan-Meier analysis and propensity score matching to strengthen the findings.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The findings underscore the critical importance of addressing smoking and alcohol consumption as key prognostic factors in the comprehensive management of OSCC patients.
Figure 4. The graphical abstract for long-term outcomes of smoker and drinker after oesophagectomy.
Introduction
Oesophageal cancer (OC) ranking seventh in incidence and sixth in mortality worldwide. In China, it is the fifth most common cancer and the fourth leading cause of cancer-related deaths.1 2 Oesophagectomy, whether combined with chemoradiotherapy or used as a standalone treatment, is a common approach for resectable oesophageal squamous cell carcinoma (OSCC).3,5 The prognosis of OC is not only related to surgical quality and treatment mode, but also influenced by various other factors.6,9 While smoking and excessive alcohol consumption are established risk factors for OSCC and impact patient survival,10,13 there is limited high-quality research on the prognosis of smokers and alcohol drinkers following oesophagectomy.
Recent research has focused on exploring the relationship between smoking, alcohol consumption, and the onset of OC, particularly OSCC.13,15 These studies have shown that smoking and alcohol consumption can lead to changes in DNA through epigenetic mechanisms,16,18 as well as the release of cytokines and chemical factors that trigger the production of inflammatory cells.19,21 This cascade of events fosters the formation of a tumour-friendly microenvironment, increasing the likelihood of cancer progression.13
A clinical study has shown that smoking patients may have higher complication rates without a clear effect on survival. However, due to the constraints of a small sample size and short median follow-up period, further high-quality research with larger sample sizes is needed to understand the impacts of smoking and alcohol consumption on patients with OSCC.22 This study seeks to explore the long-term consequences for OSCC patients who smoke and drink, providing substantial evidence for future studies in this area.
Materials and methods
Study design
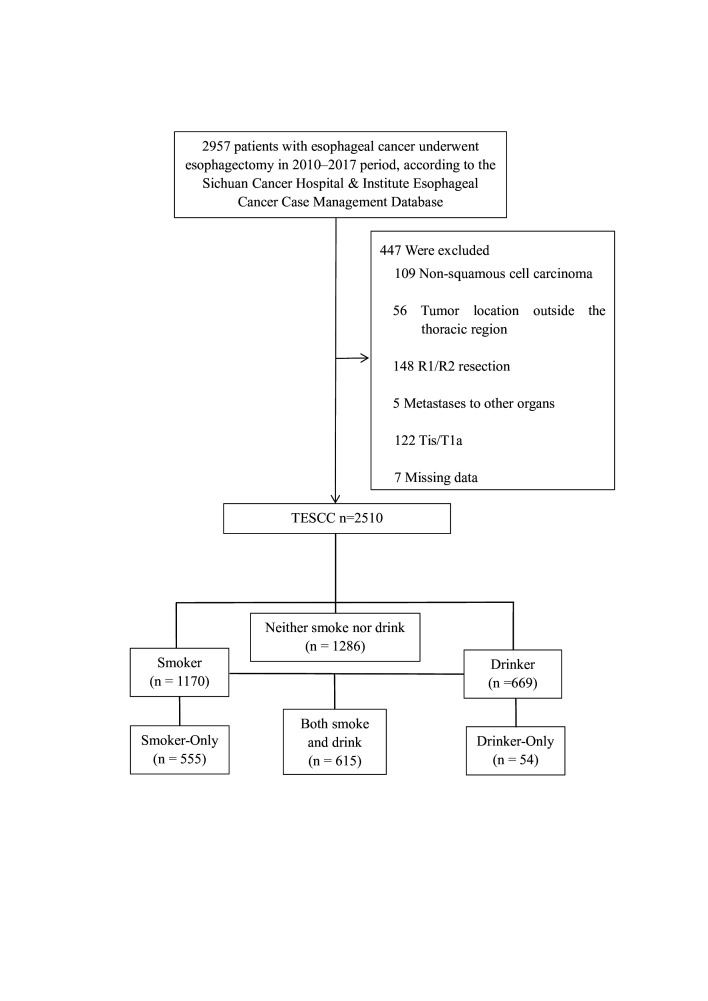
In this study, we conducted a retrospective review of data from 2510 patients with OSCC who were treated at Sichuan Cancer Hospital and Institute Esophageal Cancer Case Management Database (SCCH-ECCM Database) between January 2010 and December 2017. The data and medical records of the patients were obtained from SCCH-ECCM Database. The research is being reported in line with the Strengthening the Reporting of Observational Studies in Epidemiology guidelines.23 The research gathered demographic information on participants and tumour characteristics. Clinical stages of each patient were thoroughly reviewed by multiple experts before any treatment. Final pathological findings were analysed by two pathologists and authorised by an additional pathologist for accuracy. Disease staging followed the eighth edition tumour, node, metastasis (TNM) staging system of the Union for International Cancer Control/American Joint Committee on Cancer. Patients included in the study had undergone oesophagectomy at SCCH-ECCM Database and met specific criteria. Patients were excluded if they met specific criteria: (1) the tumour was located outside of the thoracic region, (2) pathology confirmed a non-squamous cell carcinoma, (3) evidence of distant tumour metastasis was observed, (4) R1/R2 resection was performed indicating incomplete tumour removal, (5) pTis/T1a stage, (6) they underwent preoperative neoadjuvant therapy or (7) missing data (figure 1). Patients were categorised into different groups based on their smoking and alcohol consumption history. The categories are now specified as follows: None—patients with no history of smoking or drinking. Smoker—patients who have smoked at least one cigarette per day for at least 1 year. Non-Smoker—patients who have never smoked. Smoke-Only—patients who smoke but do not drink alcohol. Drinker—patients who consume at least one alcoholic drink per day for at least 1 year. Non-Drinker—patients who have never consumed alcohol. Drinker-Only—patients who drink alcohol but do not smoke. Both—patients who both smoke and drink according to the above criteria (figure 1), Group Another refers to the group of patients with either smoking or drinking habits, but not both. Regular monitoring occurred every 3 months for the first 2 years, followed by 6-month intervals for the next 3–5 years. Overall survival (OS), measured from surgery to death or last follow-up in April 2022, was the primary outcome. Patients were moved to surgery without delays and at a similar speed across the sample.
Figure 1. Consolidated Standards of Reporting Trials diagram showing patient selection. TOSCC, thoracic oesophageal squamous cell carcinoma.
Statistical analysis
The variables were categorised and expressed as percentages for analysis. Statistical calculations were conducted using either the χ2 test or Fisher’s exact test. The OS rate was determined based on the surgery month and year up to the date of death or last follow-up. Restricted mean survival time (RMST) estimates along with 95% CIs were calculated for the four groups based on their crude and adjusted Kaplan-Meier estimates. HRs and 95% CIs were calculated. Independent risk factors for OS were identified through univariate Cox regression analyses. Cox proportional hazards regression models were applied to assess the impact of all baseline covariates on outcomes. Kaplan-Meier curves for survival analysis were generated using RStudio software (running on R V.4.3.0). Median values at specific time points within a 95% CI were compared using log-rank tests. Propensity score matching (PSM) was used to create two groups of patients, such as Smoker versus Non-Smoker, Drinker versus Non-Drinker or Drinker versus Smoker, to balance covariates. A significance level of <0.05 was set for all statistical analyses conducted using SPSS software V.23.0 (Chicago, IL, USA) and RStudio software.
Results
Patient characteristics
A total of 2510 patients diagnosed with OSCC were examined in this research. Among them, 1170 patients had a smoking history, with 555 patients reporting only smoking and not consuming alcohol. Of the 669 patients with a history of alcohol consumption, 54 patients had solely consumed alcohol and not smoked. Additionally, 1286 patients had no history of smoking or drinking, while 615 patients had a history of both smoking and drinking (figure 1). The detailed characteristics of the patients are presented in table 1. Statistical variances were noted between the Smoker and Non-Smoker groups, as well as between the Drinker and Non-Drinker groups. However, after PSM, these groups were found to be comparable (online supplemental table 1).
Table 1. Demographic characteristics of the patients.
| Characteristic | Patients (n=2510) | P value | Patients (n=2510) | P value | Patients (n=2510) | |||||
| Non-Smoker(n=1340) | Smoker(n=1170) | Non-Drinker(n=1841) | Drinker(n=669) | None(n=1286) | Smoker-Only(n=555) | Drinker-Only(n=54) | Both(n=615) | |||
| KPS | <0.001 | <0.001 | ||||||||
| ≥90 | 973 (72.6%) | 446 (38.1%) | 1161 (63.1%) | 258 (38.6%) | 953 (74.1%) | 208 (37.5%) | 20 (37.0%) | 238 (38.7%) | ||
| ≤80 | 367 (27.4%) | 724 (61.9%) | 680 (36.9%) | 411 (61.4%) | 333 (25.9%) | 347 (62.5%) | 34 (63.0%) | 377 (61.3%) | ||
| Sex | <0.001 | <0.001 | ||||||||
| Male | 898 (67.0%) | 1162 (99.3%) | 1399 (76.0%) | 661 (98.8%) | 849 (66.0%) | 550 (99.1%) | 49 (90.7%) | 612 (99.5%) | ||
| Female | 442 (33.0%) | 8 (0.7%) | 442 (24.0%) | 8 (1.2%) | 437 (34.0%) | 5 (0.9%) | 5 (9.3%) | 3 (0.5%) | ||
| Age, years | 63 (35–90) | 61 (34–85) | <0.001 | 62 (34–90) | 62 (39–85) | 0.402 | 63 (35–90) | 60 (34–84) | 64 (45–81) | 62 (39–85) |
| Tumour grade | 0.005 | 0.088 | ||||||||
| Well G1 | 250 (18.7%) | 205 (17.5%) | 334 (18.1%) | 121 (18.1%) | 240 (18.7%) | 94 (16.9%) | 10 (18.5%) | 111 (18.0%) | ||
| Moderate G2 | 519 (38.7%) | 527 (45.0%) | 745 (40.5%) | 301 (45.0%) | 498 (38.7%) | 247 (44.5%) | 21 (38.9%) | 280 (45.5%) | ||
| Poor or undifferentiated G3 | 571 (42.6%) | 438 (37.4%) | 762 (41.4%) | 247 (36.9%) | 548 (42.6%) | 214 (38.6%) | 23 (42.6%) | 224 (36.4%) | ||
| Lymphovascular invasion | 0.036 | 0.055 | ||||||||
| Yes | 216 (16.1%) | 226 (19.3%) | 308 (16.7%) | 134 (20.0%) | 203 (15.8%) | 105 (18.9%) | 13 (24.1%) | 121 (19.7%) | ||
| No | 1124 (83.9%) | 944 (80.7%) | 1533 (83.3%) | 535 (80.0%) | 1083 (84.2%) | 450 (81.1%) | 41 (75.9%) | 494 (80.3%) | ||
| Nerve invasion | 0.129 | <0.001 | ||||||||
| Yes | 245 (18.3%) | 242 (20.7%) | 326 (17.7%) | 161 (24.1%) | 230 (17.9%) | 96 (17.3%) | 15 (27.8%) | 146 (23.7%) | ||
| No | 1095 (81.7%) | 928 (79.3%) | 1515 (82.3%) | 508 (75.9%) | 1056 (82.1%) | 459 (82.7%) | 39 (72.2%) | 469 (76.3%) | ||
| Tumour location | <0.001 | <0.001 | ||||||||
| Upper | 390 (29.1%) | 197 (16.8%) | 479 (26.0%) | 108 (16.1%) | 377 (29.3%) | 102 (18.4%) | 13 (24.1%) | 95 (15.4%) | ||
| Middle | 722 (53.9%) | 645 (55.1%) | 988 (53.7%) | 379 (56.7%) | 688 (53.5%) | 300 (54.1%) | 34 (63.0%) | 345 (56.1%) | ||
| Lower | 228 (17.0%) | 328 (28.0%) | 374 (20.3%) | 182 (27.2%) | 221 (17.2%) | 153 (27.6%) | 7 (13.0%) | 175 (28.5%) | ||
| Pathological T stage | 0.002 | 0.009 | ||||||||
| T1 | 126 (9.4%) | 76 (6.5%) | 147 (8.0%) | 55 (8.2%) | 123 (9.6%) | 24 (4.3%) | 3 (5.6%) | 52 (8.5%) | ||
| T2 | 287 (21.4%) | 222 (19.0%) | 374 (20.3%) | 135 (20.2%) | 273 (21.2%) | 101 (18.2%) | 14 (25.9%) | 121 (19.7%) | ||
| T3 | 802 (59.9%) | 782 (66.8%) | 1183 (64.3%) | 401 (59.9%) | 776 (60.3%) | 407 (73.3%) | 26 (48.1%) | 375 (61.0%) | ||
| T4 | 125 (9.3%) | 90 (7.7%) | 137 (7.4%) | 78 (11.7%) | 114 (8.9%) | 23 (4.1%) | 11 (20.4%) | 67 (10.9%) | ||
| Pathological N stage | 0.018 | 0.584 | ||||||||
| N0 | 609 (45.4%) | 475 (40.6%) | 805 (43.7%) | 279 (41.7%) | 587 (45.6%) | 218 (39.3%) | 22 (40.7%) | 257 (41.8%) | ||
| N1 | 405 (30.2%) | 356 (30.4%) | 555 (30.1%) | 206 (30.8%) | 387 (30.1%) | 168 (30.3%) | 18 (33.3%) | 188 (30.6%) | ||
| N2 | 228 (17.0%) | 222 (10.9%) | 320 (17.4%) | 130 (19.4%) | 216 (16.8%) | 104 (18.7%) | 12 (22.2%) | 118 (19.2%) | ||
| N3 | 98 (7.3%) | 117 (10.0%) | 161 (8.7%) | 54 (8.1%) | 96 (7.5%) | 65 (11.7%) | 2 (3.7%) | 52 (8.5%) | ||
| TNM stage | 0.051 | 0.824 | ||||||||
| I | 125 (9.3%) | 77 (6.6%) | 148 (8.0%) | 54 (8.1%) | 121 (9.4%) | 27 (4.9%) | 4 (7.4%) | 50 (8.1%) | ||
| II | 468 (34.9%) | 397 (33.9%) | 644 (35.0%) | 221 (33.0%) | 450 (35.0%) | 194 (35.0%) | 18 (33.3%) | 203 (33.0%) | ||
| III | 600 (44.8%) | 552 (47.2%) | 839 (45.6%) | 313 (46.8%) | 577 (44.9%) | 262 (47.2%) | 23 (42.6%) | 290 (47.2%0 | ||
| IV | 147 (11.0%) | 144 (12.3%) | 210 (11.4%) | 81 (12.1%) | 138 (10.7%) | 72 (13.0%) | 9 (16.7%) | 72 (11.7%) | ||
| Thoracic surgery | <0.001 | <0.001 | ||||||||
| MIE | 702 (52.4%) | 497 (42.5%) | 803 (43.6%) | 396 (59.2%) | 667 (51.9%) | 136 (24.5%) | 35 (64.8%) | 361 (58.7%) | ||
| OO | 638 (47.6%) | 673 (57.5%) | 1038 (56.4%) | 273 (40.8%) | 619 (48.1%) | 419 (75.5%) | 19 (35.2%) | 254 (41.3%) | ||
| Abdominal surgery | <0.001 | <0.001 | ||||||||
| MIE | 559 (417%) | 405 (34.6%) | 629 (34.2%) | 335 (50.1%) | 531 (41.3%) | 98 (17.7%) | 28 (51.9%) | 307 (49.9%) | ||
| OO | 780 (58.2%) | 762 (65.1%) | 1209 (65.7%) | 333 (49.8%) | 754 (58.6%) | 455 (82.0%) | 26 (48.1%) | 307 (49.9%) | ||
| NO | 1 (0.1%) | 3 (0.3%) | 3 (0.2%) | 1 (0.1%) | 1 (0.1%) | 2 (0.4%) | 0 (0.0%) | 1 (0.2%) | ||
| Surgical approach | <0.001 | <0.001 | ||||||||
| McKeown | 1030 (76.9%) | 749 (64.0%) | 1254 (68.1%) | 525 (78.5%) | 987 (76.7%) | 267 (48.1%) | 43 (79.6%) | 482 (78.4%) | ||
| Iovr-Lewis | 292 (21.8%) | 399 (34.1%) | 560 (30.4%) | 131 (19.6%) | 281 (21.9%) | 279 (50.3%) | 11 (20.4%) | 120 (19.5%) | ||
| Sweet | 1 (0.1%) | 3 (0.3%) | 3 (0.2%) | 1 (0.1%) | 1 (0.1%) | 2 (0.4%) | 0 (0.0%) | 1 (0.2%) | ||
| Left thoracotomy and laparotomy | 17 (1.3%) | 19 (1.6%) | 24 (1.3%) | 12 (1.8%) | 17 (1.3%) | 7 (1.3%) | 0 (0.0%) | 12 (2.0%) | ||
| Clinical treatment modality | <0.001 | 0.052 | ||||||||
| Preoperative CT or RT/CRT plus surgery | 28 (2.1%) | 18 (1.5%) | 34 (1.8%) | 12 (1.8%) | 27 (2.1%) | 7 (1.3%) | 1 (1.9%) | 11 (1.8%) | ||
| Surgery alone | 817 (61.0%) | 452 (38.6%) | 904 (49.1%) | 365 (54.6%) | 784 (61.0%) | 120 (21.6%) | 33 (61.1%) | 332 (54.0%) | ||
| Surgery plus postoperative CT or RT/CRT | 495 (36.9%) | 700 (59.8%) | 903 (49.0%) | 292 (43.6%) | 475 (36.9%) | 428 (77.1%) | 20 (37.0%) | 272 (44.2%) | ||
CRT, chemoradiotherapy; CT, chemotherapy; KPSKarnofsky Performance StatusMIE, minimally invasive oesophagectomy; OO, open oesophagectomy; RT, radiotherapyTNMtumour, node, metastasis
Survival outcomes
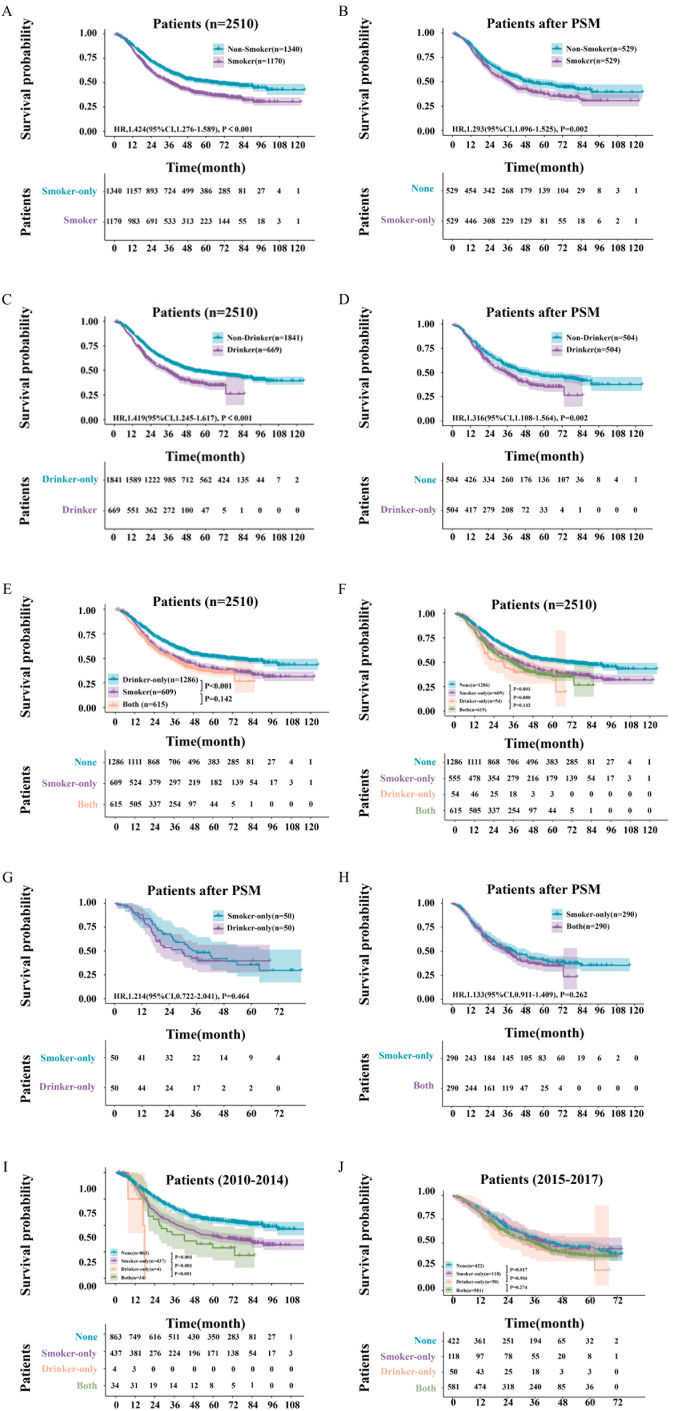
Following a median follow-up period of 64.0 months, the median OS among 2510 patients was 45.4 months (95% CI 40.5 to 50.3). Specifically, patients in the Smoker group had a median OS of 36.6 months (95% CI 33.1 to 40.1), while those in the Non-Smoker group had a median OS of 66.2 months (95% CI 53.1 to 79.2). The 1-, 3-, and 5-year OS rates were 84%, 50%, and 38% for Smoker group patients, and 88%, 62%, and 51% for Non-Smoker group patients, respectively (HR: 1.42; 95% CI 1.28 to 1.59; p<0.0001; figure 2A). After PSM, the p value was 0.002 between the Smoker and Non-Smoker groups (HR: 1.29; 95% CI 1.10 to 1.53; figure 2B). In addition, the median OS for patients in the Drinker group was 34.4 months (95% CI 29.9 to 38.9), compared with 52.0 months (95% CI 44.0 to 60.0) for those in the Non-Drinker group. The 1-, 3-, and 5-year OS rates were 83%, 48%, and 36% for Drinker group patients, and 88%, 59%, and 48% for Non-Drinker group patients, respectively (HR: 1.42; 95% CI 1.25 to 1.62; p<0.0001; figure 2C). After PSM, the p value was 0.002 between the Drinker and Non-Drinker groups (HR: 1.32; 95% CI 1.11 to 1.56; figure 2D).
Figure 2. Overall survival curve of patients. (A) Overall survival curve of patients between Smoker and Non-Smoker Group; (B) overall survival curve of patients between Smoker and Non-Smoker Group after propensity score matching (PSM); (C) overall survival curve of patients between Drinker and Non-Drinker Group; (D) overall survival curve of patients between Drinker and Non-Drinker Group after PSM; (E) overall survival curve Both smoking and drinking patients, smoking or drinking patients and None Smoking and drinking patients; (F) overall survival curve among smoking and drinking patients, smoker-only/drinker-only patients and None smoking and drinking patients after PSM; (G) overall survival curve of patients between Smoker-Only and Drinker-Only Group after PSM; (H) overall survival curve of patients between Smoker-Only and Both Group after PSM; (I, J) overall survival curve among smoking and drinking patients, smoker-only/drinker-only patients and None smoking and drinking patients. (I) Overall survival curve of patients from 2010 to 2014; (J) overall survival curve of patients from 2015 to 2017.
Subgroup analyses revealed no statistically significant difference in OS between Group Another and Group Both (p=0.142; figure 2E). Additionally, there was no statistically significant difference in OS between patients in Group Smoker-Only and Group Drinker-Only (p=0.080; figure 2F), as well as between patients in Group Drinker-Only and Group Both (p=0.142; figure 2F). Further PSM analysis showed no statistically significant difference in OS between Group Smoker-Only and Drinker-Only (p=0.464; figure 2G), and between patients in Group Smoker-Only and Group Both (p=0.262; figure 2H). Further, the patients were divided into two groups based on the median time of diagnosis in August 2014: from 2010 to 2014 and from 2015 to 2017. Kaplan-Meier curves were then conducted for survival analysis and RMST estimates for smokers and drinkers (refer to figures2 3). Due to the small number of Drink-only patients observed from 2010 to 2014 (only four patients) (see figure 2I), further analysis was not pursued for this group. The survival outcomes of patients from 2015 to 2017 were similar to the results mentioned above, showing no statistically significant difference in OS between patients in Group Smoker-Only and Group Drinker-Only (p=0.904; refer to figure 2J), as well as between patients in Group Drinker-Only and Group Both (p=0.274; refer to figure 2J).
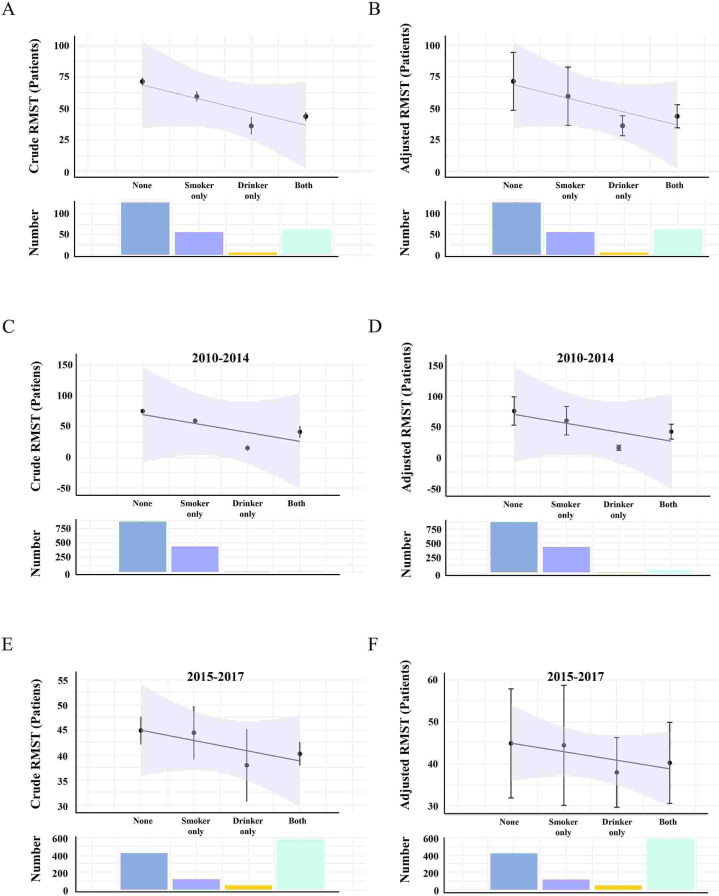
Figure 3. Restricted mean survival time (RMST) estimates patients among smoking and drinking group, smoker-only/drinker-only group and None smoking and drinking group. Black points show the RMST estimates, and whiskers show the 95% CIs. (A, B) Total patients; (C, D) patients from 2010 to 2014; (E, F) patients from 2015 to 2017.
In total, patients in Group None had a crude RMST of 71.4 (95% CI 68.3 to 74.5) months, with an adjusted RMST of 71.4 (95% CI 48.5 to 94.4) months. For patients in Group Smoker-only, the crude RMST was 59.6 (95% CI 55.5 to 63.7) months, with an adjusted RMST of 59.5 (95% CI 36.5 to 82.8) months. Patients in Group Drinker-only had a crude RMST of 36.2 (95% CI 29.3 to 43.1) months, with an adjusted RMST of 36.2 (95% CI 28.3 to 47.0) months. Finally, patients in Group Both had a crude RMST of 43.7 (95% CI 40.5 to 44.6) months, with an adjusted RMST of 43.7 (95% CI 34.6 to 52.9) months (figure 3A,B). Similar trends in RMST were observed in patients from 2010 to 2014 and 2015 to 2017 (figure 3C–F).
Risk factors
Factors significantly affecting OS after oesophagectomy were identified through univariate analyses. These factors included bad habits (p<0.001), age (p=0.004), Karnofsky Performance Status (KPS) scores (p<0.001), sex (p<0.001), thoracic surgical type (p<0.001), abdominal surgical type (p=0.024), surgical approach (p=0.004), tumour grade (p<0.001), lymphovascular invasion (p<0.001), nerve invasion (p<0.001), pathological T category (p<0.001), pathological N category (p<0.001), and TNM stage according to the eighth edition (p<0.001; table 2). Multivariate analyses indicated that bad habits (p<0.001), age (p<0.001), KPS scores (p=0.032), tumour grade (p=0.016), pathological T category (p<0.001), and pathological N category (p<0.001) were key factors influencing OS after oesophagectomy (table 2).
Table 2. Univariate and multivariate Cox regression analyses for factors affecting patient survival (n=2510).
| Univariate Cox modelHR (95% CI) | P value | Multivariate Cox modelHR (95% CI) | P value | |
| Bad habits | <0.001 | <0.001 | ||
| None | Ref. | <0.001 | Ref. | |
| Smoker-Only | 1.373 (1.202 to 1.570) | <0.001 | 1.115 (0.950 to 1.308) | 0.184 |
| Drinker-Only | 1.876 (1.313 to 2.679) | <0.001 | 1.541 (1.066 to 2.226) | 0.021 |
| Both | 1.564 (1.367 to 1.789) | <0.001 | 1.359 (1.165 to 1.586) | <0.001 |
| Age | 1.010 (1.003 to 1.017) | 0.004 | 1.018 (1.011 to 1.025) | <0.001 |
| KPS (≤80) | 1.255 (1.125 to 1.399) | <0.001 | 1.141 (1.012 to 1.286) | 0.032 |
| Sex (female) | 0.657 (0.562 to 0.767) | <0.001 | 0.852 (0.717 to 1.013) | 0.070 |
| Thoracic surgical type (OO) | 1.205 (1.079 to 1.345) | <0.001 | 0.954 (0.780 to 1.166) | 0.642 |
| Abdominal surgical type | 0.024 | 0.531 | ||
| MIE | Ref. | |||
| OO | 1.158 (1.032 to 1.298) | 0.012 | 1.081 (0.888 to 1.317) | 0.438 |
| No | 0.373 (0.052 to 2.657) | 0.325 | 0.478 (0.066 to 3.457) | 0.464 |
| Surgical approach | 0.004 | 0.139 | ||
| McKeown | Ref. | Ref. | ||
| Iovr-Lewis | 1.182 (1.051 to 1.330) | 0.005 | 1.139 (0.993 to 1.308) | 0.063 |
| Sweet | 0.360 (0.051 to 2.559) | 0.307 | ||
| Left thoracotomy and laparotomy | 1.608 (1.072 to 2.413) | 0.022 | 1.223 (0.810 to 1.846) | 0.339 |
| Tumour grade | <0.001 | 0.003 | ||
| G1 | Ref. | Ref. | ||
| G2 | 1.417 (1.199 to 1.674) | <0.001 | 1.218 (1.025 to 1.447) | 0.025 |
| G3 | 1.562 (1.323 to 1.844) | <0.001 | 1.342 (1.129 to 1.594) | <0.001 |
| Tumour location | 0.449 | |||
| Upper | Ref. | |||
| Middle | 1.087 (0.948 to 1.247) | 0.232 | ||
| Lower | 1.091 (0.928 to 1.283) | 0.293 | ||
| Lymphovascular invasion (No) | 0.572 (0.502 to 0.653) | <0.001 | 0.869 (0.755 to 1.000) | 0.051 |
| Nerve invasion (No) | 0.667 (0.585 to 0.759) | <0.001 | 0.887 (0.773 to −1.018) | 0.087 |
| Pathological T stage | <0.001 | <0.001 | ||
| T1 | Ref. | Ref. | ||
| T2 | 1.427 (1.076 to 1.894) | 0.014 | 0.953 (0.655 to 1.386) | 0.800 |
| T3 | 2.343 (1.815 to 3.026) | <0.001 | 1.397 (0.960 to 2.034) | 0.081 |
| T4 | 3.838 (2.852 to 5.165) | <0.001 | 1.653 (1.019 to 2.683) | 0.042 |
| Pathological N stage | <0.001 | <0.001 | ||
| N0 | Ref. | Ref. | ||
| N1 | 1.776 (1.546 to 2.041) | <0.001 | 1.148 (0.813 to 1.621) | 0.433 |
| N2 | 3.154 (2.721 to 3.655) | <0.001 | 1.836 (1.274 to 2.646) | 0.001 |
| N3 | 4.115 (3.436 to 4.929) | <0.001 | 1.834 (1.054 to 3.193) | 0.032 |
| TNM stage | <0.001 | 0.120 | ||
| I | Ref. | Ref. | ||
| II | 1.739 (1.284 to 2.354) | <0.001 | 1.196 (0.792 to 1.805) | 0.395 |
| III | 3.641 (2.717 to 4.880) | <0.001 | 1.784 (0.981 to 3.246) | 0.058 |
| IV | 6.980 (5.107 to 9.539) | <0.001 | 2.291 (1.082 to 4.851) | 0.030 |
| Clinical treatment modality | 0.732 | |||
| Preoperative CT or RT/CRT plus surgery | Ref. | |||
| Surgery alone | 0.863 (0.583 to 1.278) | 0.463 | ||
| Surgery plus postoperative CT or RT/CRT | 0.883 (0.596 to 1.306) | 0.532 |
CRT, chemoradiotherapy; CT, chemotherapy; KPSKarnofsky Performance StatusLN, lymph node; MIE, minimally invasive oesophagectomy; OO, open oesophagectomy; RT, radiotherapy; TNM, tumour, node, metastasis
Discussion
The study’s initial findings clearly demonstrate that patients with a history of smoking and/or drinking have poorer survival outcomes, as indicated by significantly lower median OS and annual survival rates compared with non-smoking and non-drinking patients. The Smoker group had a median OS of 36.6 months, the Drinker group had 34.4 months, while the Non-Smoker group had 66.2 months and the Non-Drinker group had 52.0 months. These differences, along with the HRs before and after PSM, highlight the negative impact of these lifestyle factors on the survival of patients with OSCC. Subgroup analyses showed no statistically significant difference in OS between patients with both smoking and drinking habits and those with only one habit. This suggests that having one bad habit does not necessarily lead to worse survival outcomes compared with having both, or it could indicate that the maximum impact of lifestyle habits on survival has already been reached with just one habit. The risk factor analysis portion of the study further confirms the influence of bad habits on OS, in addition to other clinical and pathological factors. The persistent significance of bad habits on survival even after adjusting for other variables in multivariate analyses underscores their independent impact on prognosis.
The primary approach for managing OSCC involves a comprehensive treatment regimen centred around oesophagectomy.24 Patients may opt for neoadjuvant therapy before surgery, which can include chemotherapy, chemoradiotherapy, or immunotherapy combined with chemoradiotherapy.425,27 Postoesophagectomy, the potential addition of supplementary immunotherapy is being considered.25 28 However, the most critical factor influencing patient OS is the extent of lymphadenectomy performed during oesophagectomy and the presence of metastatic lymph nodes.29,31 Various factors, such as smoking and alcohol consumption, also impact patients’ short-term and long-term prognosis.32,34
Kamarajah et al35 suggested that current smokers undergoing surgery for OC have significantly poorer long-term survival compared with ex-smokers or non-smokers. However, no survival difference was observed in patients receiving neoadjuvant therapy for adenocarcinoma.35 The study highlighted the benefits of smoking cessation, although the sample size was limited due to a lack of PSM. Similarly, Huang et al.33 proposed that emphasising alcohol control could help reduce mortality from oesophageal carcinoma.33 While smoking and alcohol consumption have long been known to be harmful to health, their relative impacts have not been directly compared.32,35 As for the impacts of smoking and alcohol consumption on survival rates, although both are known risk factors, their specific effects still require further research. Current evidence suggests that quitting smoking and limiting alcohol consumption can improve patient prognosis,33 36 comparison of our findings with those of Sun et al37 has been added, emphasising the larger sample size and different methodologies used in our study. Our research involved PSM to adjust for differences in each variable and RMST estimates smoker and drinker, and we further compared patients who only smoked with those who only drank. This comparative analysis addressed a common assumption in the field of digestive oncology regarding alcohol as the primary carcinogen and tobacco’s association with respiratory tumours. Our results indicate that both alcohol and tobacco have an equal negative impact on prognosis, with no significant statistical difference observed between the two groups of patients. But how to effectively promote these lifestyle changes in clinical practice and how these changes can be integrated with other treatment strategies to optimise patient outcomes are key areas for future research.
In summary, managing OSCC poses a multifaceted challenge that requires a comprehensive approach involving surgery, medication, and lifestyle adjustments.38 The clear and measurable impact of smoking and drinking on OS in OSCC patients underscores the importance of healthcare providers being more vigilant about the predictive value of lifestyle choices. It also suggests potential advantages in incorporating cessation support into the treatment strategies for OSCC patients. Future studies could delve deeper into the biological processes by which smoking and drinking influence disease advancement and treatment response in OSCC.
There are several limitations in our study. First, while the OS of smokers and drinkers is worse, the exact mechanism is still a topic of debate. This article focuses solely on the macroscopic outcomes. Second, although the sample size was relatively large and sourced from high-volume thoracic surgery centres, it was a retrospective analysis conducted at a single centre. Third, due to inherent heterogeneity, the sample size for statistical analysis decreased by hundreds of cases after PSM. Finally, this retrospective study concluded many smokers from rural China who used homemade tobacco with varying specifications, presenting difficulties in standardisation. Furthermore, patients consumed a variety of alcohol types such as beer, spirits, and homemade fruit or rice wines with different alcohol concentrations, further complicating standardisation efforts. Consequently, conducting a comprehensive analysis based on smoking and drinking quantities proved to be exceptionally challenging. Future studies should consider data from multicentre perspective clinical trials to further investigate the risks and mechanisms associated with smoking and alcohol consumption in patients with OSCC.
Conclusions
Our research demonstrates that patients with a history of smoking and/or drinking have significantly poorer survival outcomes compared with non-smoking and non-drinking patients. Subgroup analyses revealed no statistically significant difference in OS between patients with both smoking and drinking habits and those with only one habit, suggesting that the maximum impact of lifestyle habits on survival may have already been reached with just one habit.
supplementary material
Acknowledgements
We appreciate Yongtao Han, Xuefeng Leng, Qifeng Wang and Chenghao Wang for their financial support and everyone who contributed to the data collection.
Footnotes
Funding: The authors disclosed receipt of the following financial support for the research, authorship and publication of this article: This study was financially supported by grants from the National Key Research and Development Programme [grant number 2022YFC2403400]; the International Cooperation Projects of Science and Technology Department of Sichuan Province [grant number 24GJHZ0166]; the Sichuan Key Research and Development Project from Science and Technology Department of Sichuan Province [grant number 2024NSFSC0752, 2023YFS0044, 2023YFQ0056, 2022YFQ0008, 2023YFS0488 and 2023YFQ0055]; Key Research and Development Project of Science and Technology Department of Chengdu (2024-YF05-00797-SN); the Wu Jieping Clinical Research Projects [grant number 320.6750.2023-05-141] and Sichuan Province Clinical Key Specialty Construction Project.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Consent obtained from parent(s)/guardian(s).
Ethics approval: This study involves human participants and the study was approved by the Ethics Committee (EC) for Medical Research and New Medical Technology of Sichuan Cancer Hospital (SCCHEC-02-2022-050). The study followed the Declaration of Helsinki principles, and ethics committee consent was not required due to the study’s retrospective nature.
Data availability free text: The datasets supporting the results of the present study can be obtained from the corresponding author upon reasonable request.
Contributor Information
Kexun Li, Email: likexun@yeah.net.
Simiao Lu, Email: 1195906395@qq.com.
Changding Li, Email: 15681566680@163.com.
Wenwu He, Email: wenwu_he@126.com.
Kunyi Du, Email: sevendky@163.com.
Kun Liu, Email: 15983778937@163.com.
Chenghao Wang, Email: wch7676203@163.com.
Jialong Li, Email: lijialongmedical@163.com.
Ziwei Wang, Email: 2840009305@qq.com.
Yehan Zhou, Email: zhouyehan@scszlyy.org.cn.
Jiahua Lv, Email: lvjiahua1@scszlyy.org.cn.
Yongtao Han, Email: docyongtaohan@163.com.
Qifeng Wang, Email: littlecancer@163.com.
Xuefeng Leng, Email: doc.leng@uestc.edu.cn.
Lin Peng, Email: doclinpeng@163.com.
Data availability statement
Data are available upon reasonable request.
References
- 1.Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 2.Han B, Zheng R, Zeng H, et al. Cancer incidence and mortality in China, 2022. J Natl Cancer Cent . 2024;4:47–53. doi: 10.1016/j.jncc.2024.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang H, Liu H, Chen Y, et al. Neoadjuvant Chemoradiotherapy Followed by Surgery Versus Surgery Alone for Locally Advanced Squamous Cell Carcinoma of the Esophagus (NEOCRTEC5010): A Phase III Multicenter, Randomized, Open-Label Clinical Trial. J Clin Oncol. 2018;36:2796–803. doi: 10.1200/JCO.2018.79.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Y-Y, Dai L, Yang Y-B, et al. Long-Term Survival and Recurrence Patterns in Locally Advanced Esophageal Squamous Cell Carcinoma Patients with Pathologic Complete Response After Neoadjuvant Chemotherapy Followed by Surgery. Ann Surg Oncol. 2024;31:5047–54. doi: 10.1245/s10434-023-14809-1. [DOI] [PubMed] [Google Scholar]
- 5.Yan X, Duan H, Ni Y, et al. Tislelizumab combined with chemotherapy as neoadjuvant therapy for surgically resectable esophageal cancer: A prospective, single-arm, phase II study (TD-NICE) Int J Surg. 2022;103:106680. doi: 10.1016/j.ijsu.2022.106680. [DOI] [PubMed] [Google Scholar]
- 6.Rebernick RJ, Bell HN, Bauer TM, et al. Role of IL4 and GMCSF in Predicting Survival in Esophageal Cancer. J Am Coll Surg. 2023;236:107–15. doi: 10.1097/XCS.0000000000000446. [DOI] [PubMed] [Google Scholar]
- 7.Zhou Y, He W, Guo P, et al. Development and Validation of a Recurrence-Free Survival Prediction Model for Locally Advanced Esophageal Squamous Cell Carcinoma with Neoadjuvant Chemoradiotherapy. Ann Surg Oncol. 2024;31:178–91. doi: 10.1245/s10434-023-14308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao Y, Zhao W, Li J, et al. Effect of dietary consumption on the survival of esophageal squamous cell carcinoma: a prospective cohort study. Eur J Clin Nutr. 2023;77:55–64. doi: 10.1038/s41430-022-01194-3. [DOI] [PubMed] [Google Scholar]
- 9.Li K, Leng X, He W, et al. Resected lymph nodes and survival of patients with esophageal squamous cell carcinoma: an observational study. Int J Surg. 2023;109:2001–9. doi: 10.1097/JS9.0000000000000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soroush A, Malekzadeh R, Roshandel G, et al. Sex and smoking differences in the association between gastroesophageal reflux and risk of esophageal squamous cell carcinoma in a high-incidence area: Golestan Cohort Study. Int J Cancer. 2023;152:1137–49. doi: 10.1002/ijc.34313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katada C, Yokoyama T, Mure K, et al. Risk factors for the development of second primary esophageal squamous-cell carcinoma after endoscopic resection for esophageal squamous-cell carcinoma according to genetic polymorphisms related to alcohol and nicotine metabolism. Jpn J Clin Oncol. 2023;53:774–80. doi: 10.1093/jjco/hyad070. [DOI] [PubMed] [Google Scholar]
- 12.Jiang D, Song Q, Zhang F, et al. Prognostic significance of CCND1 amplification/overexpression in smoking patients with esophageal squamous cell carcinoma. Cancer Genet. 2023;278–279:1–8. doi: 10.1016/j.cancergen.2023.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Okawa Y, Sasagawa S, Kato H, et al. Immuno-genomic analysis reveals eosinophilic feature and favorable prognosis of female non-smoking esophageal squamous cell carcinomas. Cancer Lett. 2024;581:216499. doi: 10.1016/j.canlet.2023.216499. [DOI] [PubMed] [Google Scholar]
- 14.Yuan S, Chen J, Ruan X, et al. Smoking, alcohol consumption, and 24 gastrointestinal diseases: Mendelian randomization analysis. Elife. 2023;12:e84051. doi: 10.7554/eLife.84051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei M, Zhao L, Lv J, et al. The mediation effect of serum metabolites on the relationship between long-term smoking exposure and esophageal squamous cell carcinoma. BMC Cancer. 2021;21:415. doi: 10.1186/s12885-021-08151-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tu R, Zhong D, Li P, et al. PTPN13 rs989902 and CHEK2 rs738722 are associated with esophageal cancer. Ann Med. 2023;55:2281659. doi: 10.1080/07853890.2023.2281659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L, Du L, Xiong X, et al. Repurposing dextromethorphan and metformin for treating nicotine-induced cancer by directly targeting CHRNA7 to inhibit JAK2/STAT3/SOX2 signaling. Oncogene. 2021;40:1974–87. doi: 10.1038/s41388-021-01682-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flashner S, Shimonosono M, Tomita Y, et al. ALDH2 dysfunction and alcohol cooperate in cancer stem cell enrichment. Carcinogenesis. 2024;45:95–106. doi: 10.1093/carcin/bgad085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang F, Li G, Ning J, et al. Alcohol accumulation promotes esophagitis via pyroptosis activation. Int J Biol Sci. 2018;14:1245–55. doi: 10.7150/ijbs.24347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu S, Zhang L, Deng J, et al. A Novel Micropeptide Encoded by Y-Linked LINC00278 Links Cigarette Smoking and AR Signaling in Male Esophageal Squamous Cell Carcinoma. Cancer Res. 2020;80:2790–803. doi: 10.1158/0008-5472.CAN-19-3440. [DOI] [PubMed] [Google Scholar]
- 21.Wang G, Ye M, Zheng S, et al. Cigarette Smoke Extract induces H19 in Esophageal Squamous Cell Carcinoma in Smoking Patients: Based on A Chronic Exposed Cell Model. Toxicol Lett. 2020;333:62–70. doi: 10.1016/j.toxlet.2020.07.030. [DOI] [PubMed] [Google Scholar]
- 22.Goto H, Oshikiri T, Kato T, et al. The Influence of Preoperative Smoking Status on Postoperative Complications and Long-Term Outcome Following Thoracoscopic Esophagectomy in Prone Position for Esophageal Carcinoma. Ann Surg Oncol. 2023;30:2202–11. doi: 10.1245/s10434-022-12898-y. [DOI] [PubMed] [Google Scholar]
- 23.von Elm E, Altman DG, Egger M, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335:806–8. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ajani JA, D’Amico TA, Bentrem DJ, et al. Esophageal and Esophagogastric Junction Cancers, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2019;17:855–83. doi: 10.6004/jnccn.2019.0033. [DOI] [PubMed] [Google Scholar]
- 25.Kelly RJ, Ajani JA, Kuzdzal J, et al. Adjuvant Nivolumab in Resected Esophageal or Gastroesophageal Junction Cancer. N Engl J Med. 2021;384:1191–203. doi: 10.1056/NEJMoa2032125. [DOI] [PubMed] [Google Scholar]
- 26.Shapiro J, van Lanschot JJB, Hulshof M, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090–8. doi: 10.1016/S1470-2045(15)00040-6. [DOI] [PubMed] [Google Scholar]
- 27.Yu Y-K, Meng F-Y, Wei X-F, et al. Neoadjuvant chemotherapy combined with immunotherapy versus neoadjuvant chemoradiotherapy in patients with locally advanced esophageal squamous cell carcinoma. J Thorac Cardiovasc Surg. 2024;168:417–28. doi: 10.1016/j.jtcvs.2023.12.030. [DOI] [PubMed] [Google Scholar]
- 28.Li C, Zhao S, Zheng Y, et al. Preoperative pembrolizumab combined with chemoradiotherapy for oesophageal squamous cell carcinoma (PALACE-1) Eur J Cancer. 2021;144:232–41. doi: 10.1016/j.ejca.2020.11.039. [DOI] [PubMed] [Google Scholar]
- 29.Li K, Nie X, Li C, et al. Mapping of Lymph Node Metastasis and Efficacy Index in Thoracic Esophageal Squamous Cell Carcinoma: A Large-Scale Retrospective Analysis. Ann Surg Oncol. 2023;30:5856–65. doi: 10.1245/s10434-023-13655-5. [DOI] [PubMed] [Google Scholar]
- 30.Tachimori Y, Ozawa S, Numasaki H, et al. Efficacy of lymph node dissection by node zones according to tumor location for esophageal squamous cell carcinoma. Esophagus. 2016;13:1–7. doi: 10.1007/s10388-015-0515-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li K, Du K, Liu K, et al. Impact of two‑field or three‑field lymphadenectomy on overall survival in middle and lower thoracic esophageal squamous cell carcinoma: A single‑center retrospective analysis. Oncol Lett. 2023;25:189. doi: 10.3892/ol.2023.13774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shinozuka T, Kanda M, Shimizu D, et al. Prognostic Value of a Modified Albumin-Bilirubin Score Designed for Patients with Esophageal Squamous Cell Carcinoma After Radical Resection. Ann Surg Oncol. 2022;29:4889–96. doi: 10.1245/s10434-022-11654-6. [DOI] [PubMed] [Google Scholar]
- 33.Huang Q, Luo K, Yang H, et al. Impact of alcohol consumption on survival in patients with esophageal carcinoma: a large cohort with long-term follow-up. Cancer Sci. 2014;105:1638–46. doi: 10.1111/cas.12552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshida N, Sasaki K, Kanetaka K, et al. High Pretreatment Mean Corpuscular Volume Can Predict Worse Prognosis in Patients With Esophageal Squamous Cell Carcinoma who Have Undergone Curative Esophagectomy: A Retrospective Multicenter Cohort Study. Ann Surg Open . 2022;3:e165. doi: 10.1097/AS9.0000000000000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kamarajah SK, Madhavan A, Chmelo J, et al. Impact of Smoking Status on Perioperative Morbidity, Mortality, and Long-Term Survival Following Transthoracic Esophagectomy for Esophageal Cancer. Ann Surg Oncol. 2021;28:4905–15. doi: 10.1245/s10434-021-09720-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rayman S, Ross S, Sucandy I, et al. The effects of smoking history on robotic transhiatal esophagectomy patient outcomes. J Robot Surg. 2024;18:76. doi: 10.1007/s11701-024-01829-6. [DOI] [PubMed] [Google Scholar]
- 37.Sun P, Chen C, Zhang F, et al. Combined heavy smoking and drinking predicts overall but not disease-free survival after curative resection of locoregional esophageal squamous cell carcinoma. Onco Targets Ther. 2016;9:4257–64. doi: 10.2147/OTT.S104182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kitagawa Y, Ishihara R, Ishikawa H, et al. Esophageal cancer practice guidelines 2022 edited by the Japan esophageal society: part 1. Esophagus. 2023;20:343–72. doi: 10.1007/s10388-023-00993-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.