Figure 2. . Overview of identified real-world evidence guidance by drug development phase.
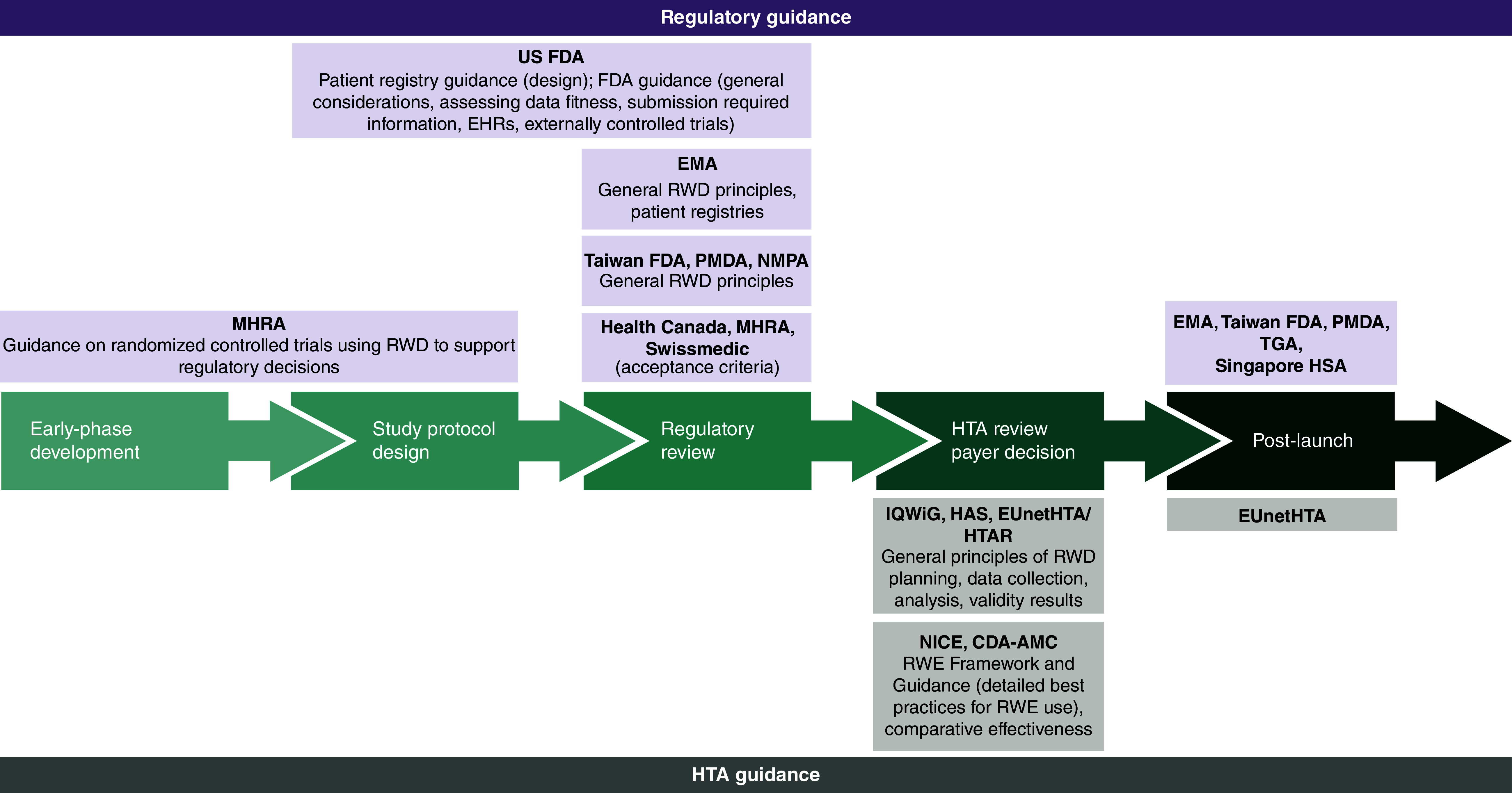
CDA-AMC: Canada's Drug Agency; EHR: Electronic health record; EMA: European Medicines Agency; EUnetHTA: European Network for Health Technology Assessment; FDA: Food and Drug Administration; HAS: Haute Autorité de Santé; HSA: Health Sciences Authority; HTA: Health technology assessment; HTAR: Health Technology Assessment Regulation; IQWiG: Institute for Quality and Efficiency in HealthCare; MHRA: Medicines and Healthcare products Regulatory Agency; NICE: National Institute for Health and Care Excellence; NMPA: National Medical Products Administration; PMDA: Pharmaceuticals and Medical Devices Agency; RWD: Real-world data; RWE: Real-world evidence; TGA: Therapeutic Goods Administration.
