Abstract
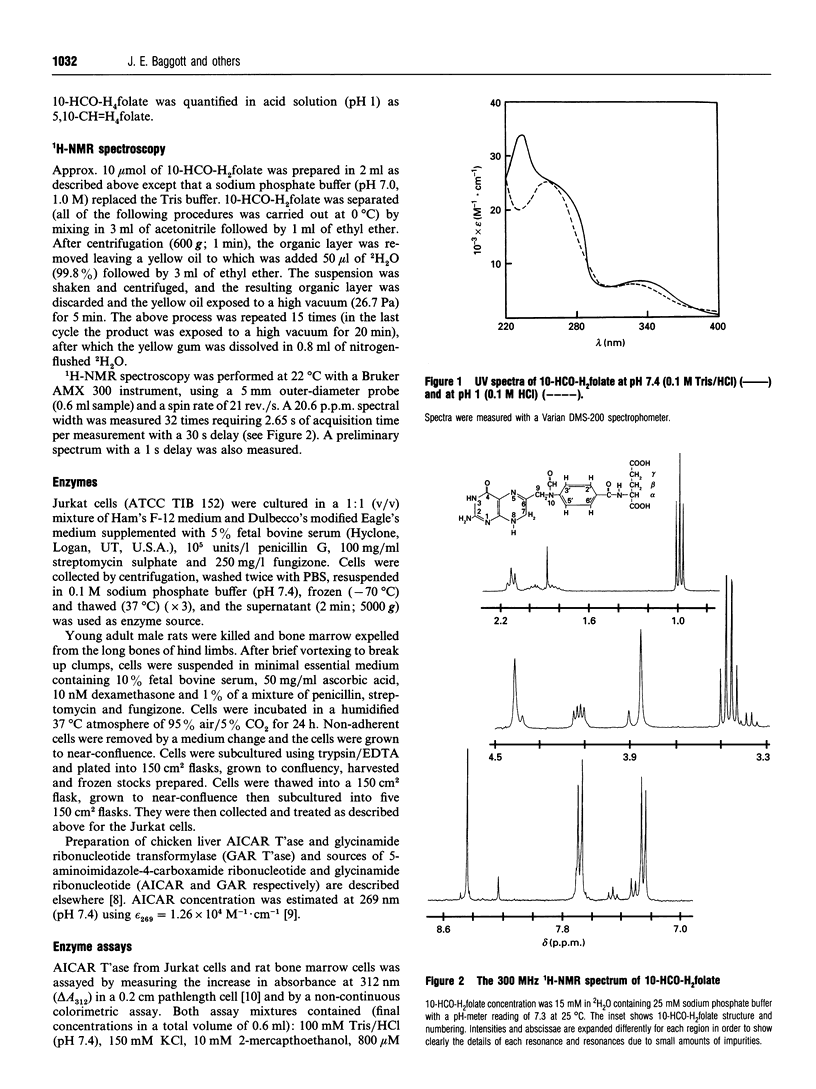
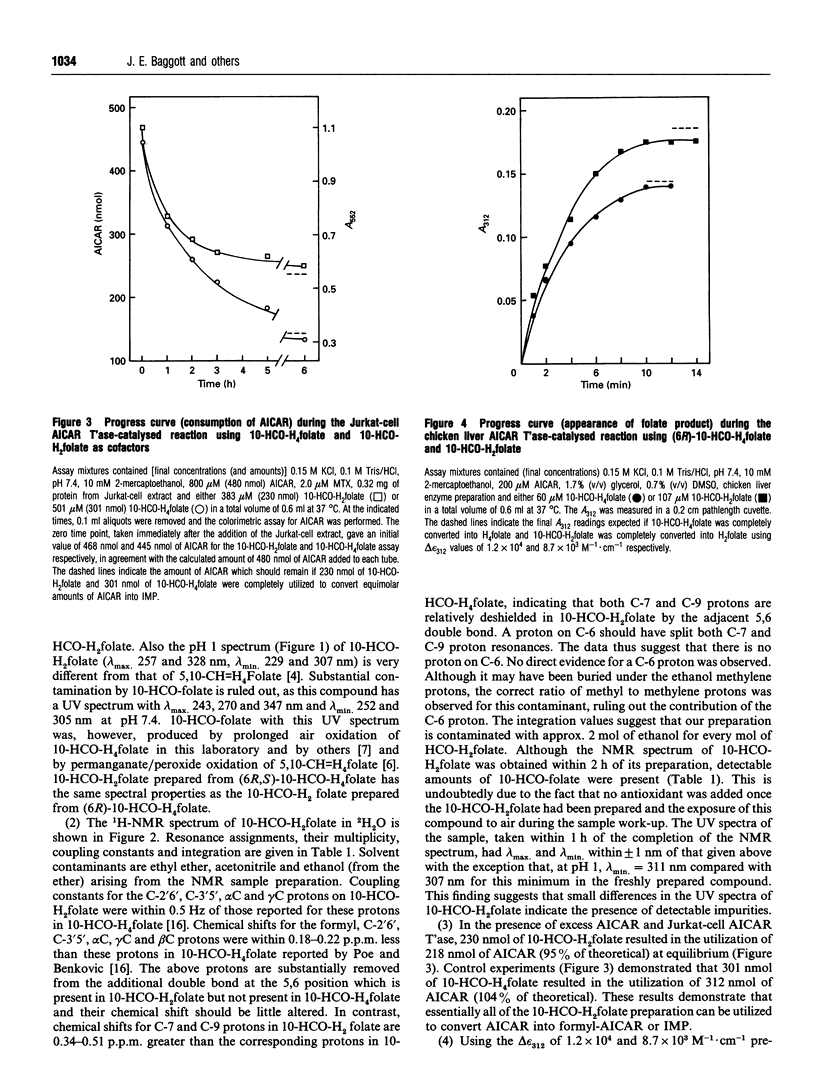
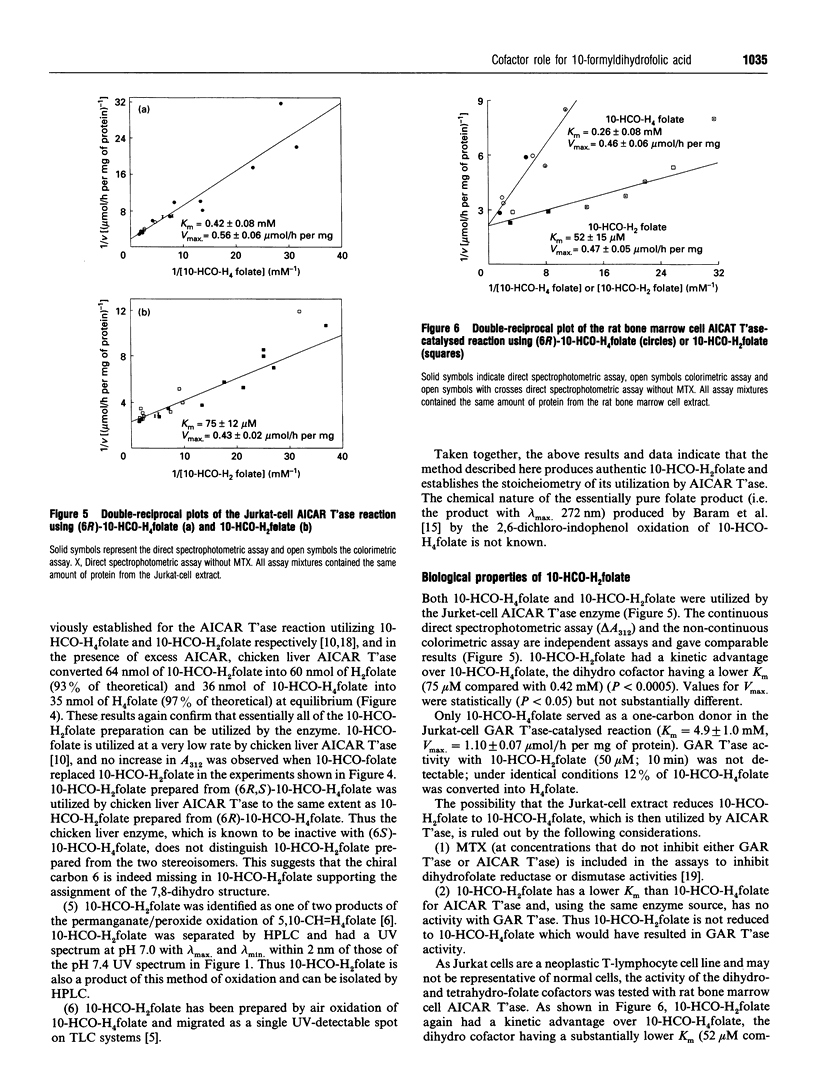
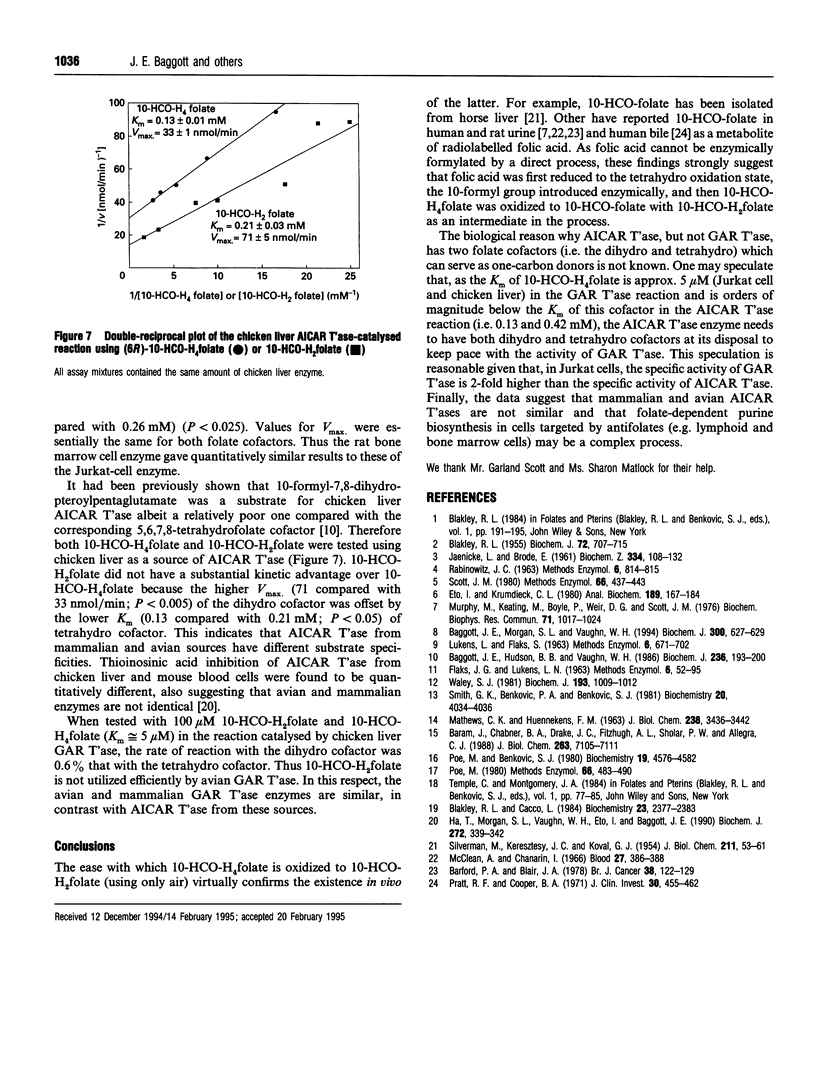
10-Formyl-7,8-dihydrofolic acid (10-HCO-H2folate) was prepared by controlled air oxidation of 10-formyl-5,6,7,8-tetrahydrofolic acid (10-HCO-H4folate). The UV spectra of the 10-HCO-H2folate preparation has lambda max. 234, 333 nm and lambda min. 301 nm at pH 7.4, and lambda max. 257, 328 nm and lambda min. 229, 307 nm at pH 1. 1H-NMR spectroscopy of 10-HCO-H2folate (in 2H2O; 300 MHz) suggested a pure compound and gave resonances for one formyl group proton, two protons on C-7 and C-9, and no evidence for a C-6 proton, which is consistent with the structure proposed. The spectral properties indicated that the 10-HCO-H2folate preparation is not appreciably contaminated with 10-HCO-H4folate, 5,10-methenyltetrahydrofolic acid (5,10-CH = H4folate) or 10-formylfolic acid (10-HCO-folate). The above data establish that the 10-HCO-H2folate prepared here is authentic. In contrast, a folate with a UV spectrum having lambda max. 272 nm and lambda min. 256 nm at pH 7, which was prepared by 2,6-dichloro-indophenol oxidation of 10-HCO-H4folate and reported to be 97% pure [Baram, Chabner, Drake, Fitzhugh, Sholar and Allegra (1988) J. Biol. Chem. 263, 7105-7111], is apparently not 10-HCO-H2folate. 10-HCO-H2folate is utilized by Jurkat-cell (human T-cell leukaemia) and chicken liver aminoimidazolecarboxamide ribonucleotide transformylase (AICAR T'ase; EC 2.1.2.3) in the presence of excess 5-amino-imidazole-4-carboxamide ribotide (AICAR) resulting in the appearance of approximately 1 mol of H2folate product for each mol of AICAR formylated. The present 10-HCO-H2folate preparation had a kinetic advantage over 10-HCO-H4folate resulting from a difference of approx. 5-fold in K(m) values when both folates were used as cofactors for Jurkat-cell and rat bone marrow AICAR T'ase. No substantial kinetic advantage was observed using chicken liver AICAR T'ase. 10-HCO-H2folate had little or no activity with Jurkat-cell or chicken liver glycinamide ribonucleotide transformylase (GAR T'ase, EC 2.1.2.2). The existence in vivo of 10-HCO-H2folate is suggested in mammals by several reports of detectable amounts of radiolabelled 10-HCO-folate in bile and urine after administration of radiolabelled folic acid.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLAKLEY R. L. The reaction of tetrahydropteroylglutamic acid and related hydropteridines with formaldehyde. Biochem J. 1959 Aug;72:707–715. doi: 10.1042/bj0720707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggott J. E., Morgan S. L., Vaughn W. H. Differences in methotrexate and 7-hydroxymethotrexate inhibition of folate-dependent enzymes of purine nucleotide biosynthesis. Biochem J. 1994 Jun 15;300(Pt 3):627–629. doi: 10.1042/bj3000627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggott J. E., Vaughn W. H., Hudson B. B. Inhibition of 5-aminoimidazole-4-carboxamide ribotide transformylase, adenosine deaminase and 5'-adenylate deaminase by polyglutamates of methotrexate and oxidized folates and by 5-aminoimidazole-4-carboxamide riboside and ribotide. Biochem J. 1986 May 15;236(1):193–200. doi: 10.1042/bj2360193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baram J., Chabner B. A., Drake J. C., Fitzhugh A. L., Sholar P. W., Allegra C. J. Identification and biochemical properties of 10-formyldihydrofolate, a novel folate found in methotrexate-treated cells. J Biol Chem. 1988 May 25;263(15):7105–7111. [PubMed] [Google Scholar]
- Barford P. A., Blair J. A. Effect of an implanted Walker tumour on metabolism of folic acid in the rat. Br J Cancer. 1978 Jul;38(1):122–129. doi: 10.1038/bjc.1978.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakley R. L., Cocco L. Dismutation of dihydrofolate by dihydrofolate reductase. Biochemistry. 1984 May 22;23(11):2377–2383. doi: 10.1021/bi00306a009. [DOI] [PubMed] [Google Scholar]
- Eto I., Krumdieck C. L. Determination of three different pools of reduced one-carbon-substituted folates. 1. A study of the fundamental chemical reactions. Anal Biochem. 1980 Nov 15;109(1):167–184. doi: 10.1016/0003-2697(80)90026-3. [DOI] [PubMed] [Google Scholar]
- Ha T., Morgan S. L., Vaughn W. H., Eto I., Baggott J. E. Detection of inhibition of 5-aminoimidazole-4-carboxamide ribotide transformylase by thioinosinic acid and azathioprine by a new colorimetric assay. Biochem J. 1990 Dec 1;272(2):339–342. doi: 10.1042/bj2720339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAENICKE L., BRODE E. [Research on monocarbon compounds. I. The tetrahydrofolate formylase from pigeon liver. Purification and mechanism]. Biochem Z. 1961;334:108–132. [PubMed] [Google Scholar]
- MATHEWS C. K., HUENNEKENS F. M. FURTHER STUDIES ON DIHYDROFOLIC REDUCTASE. J Biol Chem. 1963 Oct;238:3436–3442. [PubMed] [Google Scholar]
- McLean A., Chanarin I. Urinary excretion of 5-methyl-tetrahydrofolate in man. Blood. 1966 Mar;27(3):386–388. [PubMed] [Google Scholar]
- Murphy M., Keating M., Boyle P., Weir D. G., Scott J. M. The elucidation of the mechanism of folate catabolism in the rat. Biochem Biophys Res Commun. 1976 Aug 23;71(4):1017–1024. doi: 10.1016/0006-291x(76)90756-7. [DOI] [PubMed] [Google Scholar]
- Poe M., Benkovic S. J. 5-Formyl- and 10-formyl-5,6,7,8-tetrahydrofolate. Conformation of the tetrahydropyrazine ring and formyl group in solution. Biochemistry. 1980 Sep 30;19(20):4576–4582. doi: 10.1021/bi00561a006. [DOI] [PubMed] [Google Scholar]
- Poe M. PMR characteristics of folic acid and analogs. Methods Enzymol. 1980;66:483–490. doi: 10.1016/0076-6879(80)66491-x. [DOI] [PubMed] [Google Scholar]
- Pratt R. F., Cooper B. A. Folates in plasma and bile of man after feeding folic acid--3H and 5-formyltetrahydrofolate (folinic acid). J Clin Invest. 1971 Feb;50(2):455–462. doi: 10.1172/JCI106513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILVERMAN M., KERESZTESY J. C., KOVAL G. J. Isolation of N-10-formyfolic acid. J Biol Chem. 1954 Nov;211(1):53–61. [PubMed] [Google Scholar]
- Scott J. M. Thin-layer chromatography of pteroylmonoglutamates and related compounds. Methods Enzymol. 1980;66:437–443. doi: 10.1016/0076-6879(80)66486-6. [DOI] [PubMed] [Google Scholar]
- Smith G. K., Benkovic P. A., Benkovic S. J. L(-)-10-Formyltetrahydrofolate is the cofactor for glycinamide ribonucleotide transformylase from chicken liver. Biochemistry. 1981 Jul 7;20(14):4034–4036. doi: 10.1021/bi00517a013. [DOI] [PubMed] [Google Scholar]
- Waley S. G. An easy method for the determination of initial rates. Biochem J. 1981 Mar 1;193(3):1009–1012. doi: 10.1042/bj1931009. [DOI] [PMC free article] [PubMed] [Google Scholar]


