Abstract
A 31-year-old female presented to our hospital with sudden headache and altered consciousness. Computed tomography showed left acute subdural hematoma, and digital subtraction angiography revealed a small aneurysm on the left distal posterior cerebral artery. Coil embolization was conducted, and the patient was discharged with no neurological deficits. However, two weeks later, she presented with complete left hemiplegia and with the National Institutes of Health Stroke Scale of 20. Magnetic resonance angiography showed the occlusion of right middle cerebral artery, and the Diffusion-Weighted Imaging-Alberta Stroke Program Early Computed Tomography Score was four. Mechanical thrombectomy was conducted. Complete recanalization was achieved, and the patient recovered favorably. Although she showed no symptoms of infection such as fever throughout the treatment of aneurysm and thrombectomy, her blood culture was positive for streptococcus mitis. Furthermore, the thrombus retrieved by thrombectomy showed bacterial mass, and transesophageal echocardiography (TEE) showed vegetation on the mitral valve that could not be detected by transthoracic echocardiography. Therefore, the patient was diagnosed with infective endocarditis (IE). She was administered penicillin for 6 weeks and was discharged with no neurological deficits. When treating young patients with small aneurysms in rare locations, IE should be suspected, and blood culture and TEE should be conducted, even when there are no obvious symptoms of systemic infection.
Keywords: coil embolization, distal posterior cerebral artery aneurysm, embolic cerebral infarction, infective endocarditis, mechanical thrombectomy
Introduction
Aneurysms on the posterior cerebral artery (PCA) are uncommon, representing only 1% of all intracranial aneurysms.1) Distal PCA aneurysms are even rarer.2) Some reported etiologies of PCA aneurysms are trauma, infection, and other associated disorders such as moyamoya disease, arteriovenous malformations, polycystic kidney disease, Ehlers-Danlos syndrome, and fibromuscular disease.3) Here, we describe a rare case of a young patient who presented with acute subdural hematoma (ASDH) due to a ruptured aneurysm on distal PCA and later developed middle cerebral artery (MCA) occlusion, which led to the diagnosis of infective endocarditis (IE).
Case Report
A 31-year-old female with no past medical history presented with sudden headache and altered consciousness. Computed tomography (CT) showed left ASDH that spread along the tentorium of cerebelli (Fig. 1a, b). There was no prior trauma. Even though contrast-enhanced CT did not reveal any aneurysms or vascular malformations, digital subtraction angiography (DSA) was conducted to detect occult vascular malformations that may have caused the left ASDH. Left vertebral artery angiogram showed an aneurysm on the left distal PCA, and it was 3 mm in size (Fig. 1c, d). ASDH was caused due to the rupture of this left distal PCA aneurysm. The hematological examination upon admission indicated a mild elevation in the white blood cell (WBC) count at 16,650 and C-reactive protein (CRP) level at 1.9.
Fig. 1.
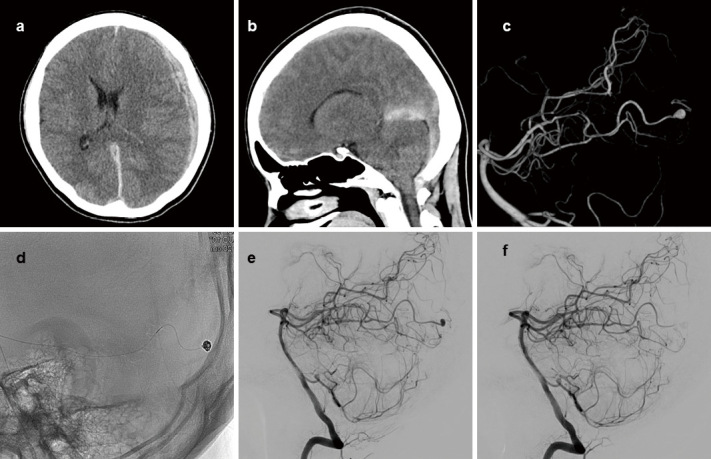
Axial (a) and sagittal (b) images of head CT show ASDH on the left side and along the tentorium of cerebelli. 3D image (c) and lateral view (e) of the left vertebral artery angiogram show a small aneurysm on the left distal PCA. Coils were used to embolize the aneurysm (d). Lateral view of the postoperative vertebral artery angiogram (f) shows no residual aneurysm.
Coil embolization of the aneurysm and parent artery occlusion of the distal PCA were conducted on the eighth day after admission. Prior to treatment, 3,000 units of heparin were administered. Coil embolization was conducted by simple technique, using a single microcatheter, Marathon (Medtronic, Minneapolis, Minnesota, USA). iED coil Complex SilkySoft 3 mm × 6 cm (Kaneka Medical Products, Minato-ku, Tokyo, Japan) was used for framing, followed by iED coil Complex SilkySoft 2 mm × 8 cm, which was used for filling the aneurysm. 33% n-butyl-2-cyanoacrylate (NBCA) was injected to achieve complete embolization of the aneurysm and occlusion of the distal PCA. (Fig. 1e). Postoperative DSA showed no residual aneurysm (Fig. 1f), and PCA territory distal to the aneurysm was perfused by pial collateral vessels. Magnetic resonance imaging (MRI) showed no ischemic complications. MRI T2*-weighted images showed no findings suggestive of multiple microbleeds. The patient was discharged on the 27th day after presentation with no neurological deficits. Throughout the treatment, the patient remained afebrile, and her hematological examination at discharge showed WBC counts of 6,960 and CRP level of 0.9; therefore, infectious etiology was not strongly suspected. Considering the possibility of IE, transthoracic echocardiography (TTE) was conducted, revealing no abnormalities. No further examinations to rule out IE, such as blood culture or transesophageal echocardiography (TEE), were considered necessary at this point due to the lack of symptoms of systemic infection.
Two weeks later, the patient presented at the emergency room again with complete left hemiplegia, and the National Institutes of Health Stroke Scale (NIHSS) was 20. MRI taken 1.5 hours after the onset revealed cerebral ischemia in the right MCA area and proximal right MCA occlusion (Fig. 2a, b). The Diffusion-Weighted Imaging-Alberta Stroke Program Early CT Score (DWI-ASPECTS) was 4. Due to the history of intracranial hemorrhage, intravascular tissue-type plasminogen activator could not be administered. Mechanical thrombectomy was conducted. After three passes of combined technique using the stent retriever assisted vacuum-locked extraction (SAVE) technique,4) tough thrombus persisted. Therefore, the stent was inserted into the aspiration catheter, which was vacuumed to obtain tight hold of the thrombus, and the thrombus was retrieved by the aspiration catheter. Complete recanalization of Thrombolysis in Cerebral Infarction grade 3 was attained 3 hours and 47 minutes after the onset (Fig. 2c, d). Complete hemiplegia gradually improved, and by the next day, the patient was able to move her left extremities. Only slight numbness in her left hand persisted, and the NIHSS improved to 1.
Fig. 2.
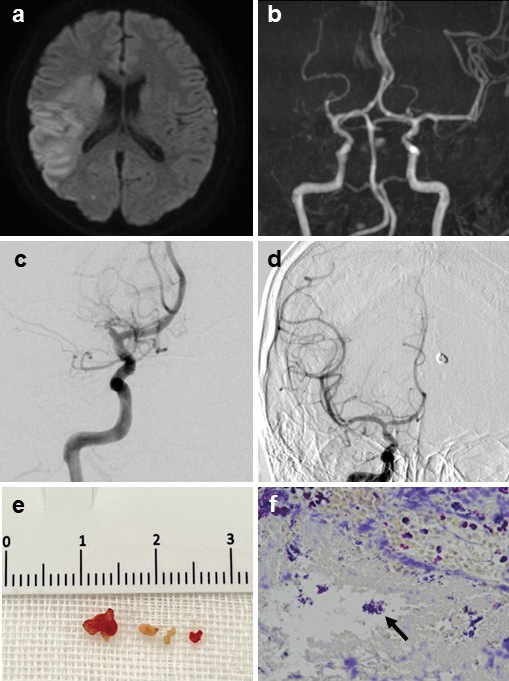
Diffusion-Weighted Imaging of MRI (a) shows cerebral ischemia in the right MCA area, and magnetic resonance angiography (b) shows proximal right MCA occlusion. Preoperative right internal carotid artery angiogram (c) shows right MCA occlusion, and postoperative right internal carotid artery angiogram (d) shows complete recanalization. Tough thrombus (e) was retrieved, and pathology (f) shows gram-positive bacterial mass. The arrow indicates the bacterial mass.
The etiology of the ruptured distal aneurysm and large vessel occlusion was investigated. As for the aneurysmal rupture, there was no prior trauma that may have caused traumatic aneurysm. Upon the first admission, the patient had no fever or bacterial infection, and TTE revealed no abnormalities; therefore, the possibility of infectious intracranial aneurysm (IIA) was considered low. During the second admission, etiology of cerebral ischemia was investigated thoroughly. All the markers for autoimmune disease were negative, and proteins S and C were in the normal range. Holter electrocardiogram showed no abnormal arrhythmia. TTE was conducted for the second time, but it was within normal limits except for a subtle mitral regurgitation. Even though the patient was also afebrile throughout the second hospitalization, we highly suspected IE due to the history of distal PCA aneurysmal rupture and MCA occlusion. Therefore, we conducted blood culture, which turned out positive for streptococcus mitis. TEE showed vegetation (9 mm) on the mitral valve and revealed mild mitral valve reflux, leading to the definitive diagnosis of IE. The thrombus that was retrieved during mechanical thrombectomy showed gram-positive bacterial mass (Fig. 2e, f). A thorough physical examination following the IE diagnosis revealed splinter hemorrhage. IE was the possible cause of the distal PCA aneurysm and septic embolism. Furthermore, IE was not diagnosed during the first hospitalization because there were no symptoms such as fever or cardiac murmur that would indicate it, and TTE was not efficient in revealing vegetation. The patient had no medical history that might have suggested IE such as dental treatment or intravenous treatment prior to her presentation of ASDH and the entry root of the bacteria remained unidentified.
The patient was treated with penicillin for 6 weeks, with no antithrombotic drugs. Cardiac surgery was not required because the vegetation was smaller than 10 mm in size, her blood culture taken 3 days after the initiation of the antibacterial treatment was negative, and she showed no symptoms of cardiac failure or valve destruction. TEE conducted 2 weeks after the initial TEE showed that the vegetation decreased in size to 3 mm. She was discharged 6 weeks after the presentation with no neurological deficits. Follow-up CT angiography did not show any de novo aneurysms.
Discussion
We experienced a case of ruptured distal PCA aneurysm followed by MCA occlusion due to IE. The rarity of this case is reflected in the fact that even though the patient did not show any IE symptoms during the two hospitalizations, her blood culture was positive, and TEE revealed vegetation, leading to the IE diagnosis.
IE is an uncommon disease that involves infection of the endocardial surface of the heart. The presentation of IE is polymorphic, which accounts for the difficulties in diagnosis.5) Typical presentations of IE are systemic signs of infection such as fever, malaise, cardiac murmurs, and absent distal pulses.5-7) Neurological complications include ischemic stroke, cerebral hemorrhage due to ruptured IIA, meningitis, and cerebral abscess.5) The IE diagnosis should be considered when stroke or embolic complication is associated with fever.8)
IIAs are distinct from other intracranial aneurysms because of the infectious entity, occurrence in younger people, multiplicity, small size, and distal location.6) IIAs represent approximately between 0.5% and 6.5% of all aneurysms, and their mortality is as high as 18.5%.9)
There are previous reports of IIAs, but prior systemic infections are common in such cases.10-13) In our case, we were unable to diagnose IE when the patient presented with ASDH due to the lack of systemic symptoms suggestive of infection and no risk factors of IE. Had we achieved the diagnosis of IE after the rupture of the distal PCA aneurysm, MCA embolism could have been prevented. When treating small aneurysms in distal location in young patients, it is necessary to suspect IE and conduct blood culture and TEE for definitive diagnosis, even if there are no obvious symptoms of systemic infection.
Antimicrobial medication is the first-line treatment for IIAs.6) As for ruptured IIAs, endovascular or surgical treatment to prevent rerupture of IIAs is essential. An increasing number of cases of IIAs are treated safely and less invasively by endovascular treatment.14,15) In our case, trapping of the aneurysm was considered as the second-line treatment if the aneurysm could not be effectively embolized by endovascular treatment. Endovascular treatment was effective and less invasive; however, there was a disadvantage of not allowing histological diagnosis. If trapping of the aneurysm was conducted, the aneurysm would have been pathologically investigated, and the bacterial bodies would have been detected earlier. Clinicians need to be more suspicious of IE and perform various tests to investigate the infectious etiology when conducting endovascular treatment to distal aneurysms.
Conclusion
We experienced a rare case of IE that presented with ruptured distal PCA aneurysm followed by MCA occlusion. When treating young patients with small aneurysms present in rare locations, their entity needs to be investigated thoroughly, and blood culture examination and TEE should be considered, even when the obvious symptoms of systemic infection are absent.
Funding
We declare that this research received no financial support from any organizations.
Availability of Data and Material
All relevant data and materials regarding this study are available upon request from the corresponding author.
Authors' Contributions
All authors have contributed to the diagnosis and treatment of the patient. The first author contributed substantially to drafting the case report. All authors have reviewed and approved the final version of the manuscript for publication.
Ethics Approval
This study was approved by Japan Community Health Care Organization Tokyo Shinjuku Medical Center ethics committee. (Approval number: R5-29)
Consent to Participate
A written consent was provided by the patient to be reported in this case report.
Consent for Publication
A written consent for publication was provided by the patient to appear in publication.
Conflicts of Interest Disclosure
We have no conflicts of interest or competing interests that could influence the interpretation of this study.
References
- 1).Ciceri EF, Klucznik RP, Grossman RG, et al. : Aneurysms of the posterior cerebral artery: Classification and endovascular treatment. Am J Neuroradiol 22: 27-34, 2001 [PMC free article] [PubMed] [Google Scholar]
- 2).Essibayi MA, Oushy SH, Keser Z, Lanzino G: Natural history and management of posterior cerebral artery aneurysms: a systematic review and meta-analysis of individual patient data. Neurosurg Rev 45: 3595-3608, 2022 [DOI] [PubMed] [Google Scholar]
- 3).Wiebers DO, Whisnant JP, Huston J, et al. : Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 362: 103-110, 2003 [DOI] [PubMed] [Google Scholar]
- 4).Maus V, Henkel S, Riabikin A, et al. : The SAVE technique: large-scale experience for treatment of intracranial large vessel occlusions. Clin Neuroradiol 29: 669-676, 2019 [DOI] [PubMed] [Google Scholar]
- 5).Iung B, Duval X: Infective endocarditis: innovations in the management of an old disease. Nat Rev Cardiol 16: 623-635, 2019 [DOI] [PubMed] [Google Scholar]
- 6).Kannoth S, Thomas SV: Intracranial microbial aneurysm (infectious aneurysm): current options for diagnosis and management. Neurocrit Care 11: 120-129, 2009 [DOI] [PubMed] [Google Scholar]
- 7).Baddour LM, Wilson WR, Bayer AS, et al. : Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation 111: e394-e434, 2005 [DOI] [PubMed] [Google Scholar]
- 8).Deprèle C, Berthelot P, Lemetayer F, et al. : Risk factors for systemic emboli in infective endocarditis. Clin Microbiol and Infection 10: 46-53, 2004 [DOI] [PubMed] [Google Scholar]
- 9).Alawieh A, Chaudry MI, Turner RD, Turk AS, Spiotta AM: Infectious intracranial aneurysms: a systematic review of epidemiology, management, and outcomes. J NeuroIntervent Surg 10: 708-716, 2018 [DOI] [PubMed] [Google Scholar]
- 10).Akimoto K, Yanaka K, Nakamura K, et al. : Simultaneous intracerebral and subarachnoid hemorrhages caused by multiple infectious intracranial aneurysms treated endovascularly and by microsurgical clipping: illustrative case. J Neurosurg: Case Lessons 3: CASE21685, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Ando K, Hasegawa H, Kikuchi B, et al. : Treatment strategies for infectious intracranial aneurysms: report of three cases and review of the literature. Neurol Med Chir 59: 344-350, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Yanagawa T, Ikeda S, Yoshitomi S, Shibata A, Ikeda T: A case of infectious intracranial aneurysm that formed and ruptured within a few days after occlusion of the proximal middle cerebral artery by infective endocarditis. Surg Neurol Int 14: 193, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Musthafa I, Kandel D, Rajlawot K, Neupane NP, Sitaula A: Infective endocarditis complicated by cerebral abscess and mycotic intracranial aneurysm: a case report. Radiol Case Rep 17: 3690-3693, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Qin X, Xu F, Maimaiti Y, et al. : Endovascular treatment of posterior cerebral artery aneurysms: a single center's experience of 55 cases. J Neurosurg 126: 1094-1105, 2017 [DOI] [PubMed] [Google Scholar]
- 15).Matsumura H, Kato N, Fujiwara Y, et al. : Endovascular treatments for posterior cerebral artery aneurysms and vascular insufficiency of fetal-type circulation after parent artery occlusion. J Clin Neurosci 32: 41-46, 2016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data and materials regarding this study are available upon request from the corresponding author.


