Abstract
Introduction
The third-generation antibody-drug conjugates (ADC), trastuzumab deruxtecan (T-DXd) and sacituzumab govitecan (SG), recently obtained approval for metastatic breast cancer treatment across various subtypes and therapeutic contexts.
Materials and Methods
This retrospective, multicentric study evaluated real-world tolerability, feasibility and efficacy in a pre-treated, real-world cohort at three major German breast cancer centers.
Results
125 patients treated with T-DXd or SG from November 2020 to June 2023 were included (T-DXd: 77 patients; SG: 48 patients). The median treatment duration was 6.0 months for T-DXd and 3.5 months for SG therapy, with a median follow-up duration of 10.4 months for T-DXd (95% CI: 8.4–11.6) and 11.8 months for SG (95% CI: 8.0–14.4). Severe neutropenia (CTC ≥ III°) occurred in 33.3% during SG therapy, with a numerical reduction observed following primary, prophylactic use of G-CSF. T-DXd-associated pneumonitis occurred in 8 out of 77 patients (10.4 %). Median progression-free survival (mPFS) was 8.6 months (95% CI: 5.8–12.4) with T-DXd (HER2+: 10.8; HER2-low: 4.7) and 4.9 months (95% CI: 2.8–6.3) with SG (TNBC 4.9; HR+/HER2−: not reached). Median overall survival (OS) was 23.8 months (95% CI: 16.1–not estimable) with T-DXd (HER2+: 27.1; HER2-low: not reached), and 12.4 months (95% CI: 8.7–not estimable) with SG therapy (TNBC: 12.4, HR+/HER2−: not reached). 95.7% of the protocol-specified, therapeutic dose was administered for T-DXd and 89.6% for SG.
Conclusion
Overall, this indicates good feasibility, tolerability, and effectiveness of ADC therapies in the real-world setting.
Keywords: oncology, breast cancer, systemic therapy, ADCs, sacituzumab govitecan, trastuzumab deruxtecan, real-world evidence
Zusammenfassung
Einleitung
Trastuzumab-Deruxtecan (T-DXd) und Sacituzumab-Govitecan (SG) sind Antikörper-Wirkstoff-Konjugate (ADCs) der 3. Generation, die vor Kurzem zur Behandlung von metastatischem Brustkrebs über mehrere Subtypen hinweg und in verschiedenen therapeutischen Zusammenhängen zugelassen wurden.
Material und Methoden
Ziel dieser retrospektiven multizentrischen Studie war es, die Real-World-Daten über die Verträglichkeit, Umsetzbarkeit und Wirksamkeit dieser Wirkstoffe in einer vorbehandelten Real-World-Kohorte in 3 großen deutschen Brustkrebszentren zu bewerten.
Ergebnisse
Eingeschlossen wurden 125 Patientinnen, die zwischen November 2020 und Juni 2023 mit T-DXd oder SG behandelt wurden (T-DXd: 77 Patientinnen; SG: 48 Patientinnen). Die mediane Behandlungsdauer betrug 6,0 Monate für eine T-DXd- und 3,5 Monate für eine SG-Therapie mit einer medianen Nachbeobachtungszeit von 10,4 Monaten für T-DXd (95%-KI: 8,4–11,6) und 11,8 Monaten für SG (95%-KI: 8,0–14,4). 33,3% der Patientinnen entwickelten eine schwere Neutropenie (CTC ≥ III°) im Verlauf der SG-Therapie, wobei eine numerische Reduktion nach dem primären prophylaktischen Einsatz von G-CSF beobachtet wurde. Bei 8 von 77 Patientinnen (10,4 %) trat eine T-DXd-bedingte Pneumonitis auf. Das mediane progressionsfreie Überleben (mPFÜ) betrug 8,6 Monate (95%-KI: 5,8–12,4) mit T-DXd (HER2+: 10,8; HER2-low: 4,7) und 4,9 Monate (95%-KI: 2,8–6,3) mit SG (TNBC 4,9; HR+/HER2−: nicht erreicht). Das mediane Gesamtüberleben (GÜ) betrug 23,8 Monate (95%-KI: 16,1–nicht schätzbar) mit einer T-DXd-Therapie (HER2+: 27,1; HER2-low: nicht erreicht) und 12,4 Monate (95%-KI: 8,7–nicht schätzbar) mit einer SG-Therapie (TNBC: 12,4, HR+/HER2−: nicht erreicht). Verabreicht wurden 95,7% der im Protokoll vorgegebenen therapeutischen T-DXd-Dosis bzw. 89,6% der vorgegebenen SG-Dosis.
Schlussfolgerung
Insgesamt weisen die Daten auf eine gute Umsetzbarkeit, Wirksamkeit und Verträglichkeit von ADC-Therapien in einer realen Umgebung hin.
Schlüsselwörter: Onkologie, Brustkrebs, systemische Therapie, ADCs, Sacituzumab-Govitecan, Trastuzumab-Deruxtecan, Real-World Evidence
Introduction
Metastatic breast cancer (mBC) remains a major challenge for healthcare professionals and patients alike, with a significant impact on quality of life and survival outcomes 1 . Despite advancements in therapy and prognosis, mBC remains incurable, making the maintenance of quality of life, alongside the extension of survival, an important therapeutic goal. Antibody-drug conjugates (ADCs) offer effective treatment with favorable tolerability, by combining monoclonal antibodies’ specificity with chemotherapeutic drugs’ cytotoxic effects 2 .
The third-generation ADCs, trastuzumab deruxtecan (T-DXd) and sacituzumab govitecan (SG), have recently gained approval for the treatment of mBC across multiple subtypes and therapeutic contexts, supported by robust phase III study findings (DESTINY-Breast03 [DB03: T-DXd; HER2+ mBC], DESTINY-Breast04 [DB04: T-DXd; HER2-low mBC], ASCENT [SG; triple-negative mBC], and TROPiCS-02 (SG; HR+/HER2− mBC]) 3 4 5 6 .
SG is an ADC in which SN-38, an active metabolite of irinotecan, is conjugated through a cleavable linker to a humanized monoclonal antibody against the trophoblast cell surface antigen-2 (Trop-2) 7 . Upon binding to Trop-2, the ADC undergoes internalization, leading to SN-38 release within tumor cells and the tumor microenvironment, due to the cleavable linker 8 .
In the IMMU-132 basket trial (NCT01631552), SG demonstrated promising results in treatment of metastatic, triple-negative breast cancer (mTNBC) therapy, achieving a median progression-free survival (PFS) of 5.5 months and a median overall survival (OS) of 13.0 months, following at least two prior chemotherapies in the metastatic setting 9 10 . The ASCENT trial compared SG with treatment of physician’s choice (TPC) in mTNBC patients who had received at least two prior systemic therapies. Significant improvements with SG compared to TPC were shown (median PFS: 4.8 months vs. 1.7 months; median OS: 11.8 months vs. 6.9 months) 11 , leading to SG’s approval for mTNBC patients who had undergone two or more prior systemic therapy lines, with at least one in the metastatic setting 12 13 . In HR+/ HER2− mBC, promising results emerged from a phase I/II basket trial (NCT01631552) 14 , further supported by the subsequent phase III TROPiCS-02 trial 15 16 . TROPiCS-02 revealed a median PFS of 5.5 months with SG, versus 4.0 months with TPC, and a median OS of 14.4, versus 11.2 months 15 16 , resulting in SG’s approval for HR+/HER2− mBC, following endocrine therapy and two or more additional, systemic therapies in the advanced setting.
T-DXd is a second, third-generation ADC, comprising a humanized monoclonal HER2 antibody, linked via a cleavable linker to a topoisomerase inhibitor payload 17 . In HER2+ mBC patients, who had received a median number of six lines of treatment, T-DXd demonstrated efficacy, with a median PFS of 19.4 months in the DESTINY-Breast01 trial (NCT03248492) 18 19 . In the phase III DESTINY-Breast03 trial (NCT03529110), second-line T-DXd therapy showed superior PFS and OS, compared to T-DM1 in patients with HER2+ mBC who had progressed after first-line therapy (PFS: 28.8 months vs. 6.8 months) 3 20 . Results from in vitro studies demonstrated that T-DXd exhibits activity even in cells with low HER2 expression 21 . The DESTINY-Breast04 trial demonstrated significantly prolonged PFS and OS, compared to physician’s choice chemotherapy (10.1 vs. 5.4 months and 23.4 vs. 16.8 months) in patients with HER2-low mBC who had received one prior line of chemotherapy. The DESTINY-Breast05 trial (NCT04622319) is currently comparing post-neoadjuvant treatment with T-DXd versus T-DM1 in patients with high-risk HER2-positive eBC.
While clinical trials are pivotal for establishing efficacy, real-world evidence enables the evaluation of treatment performance in real-life scenarios, assisting clinicians in making well-informed decisions, customized to the patient’s individual needs. As recent and upcoming clinical trial results suggest widespread use of ADCs, this study aims to evaluate the efficacy, tolerability, and feasibility of ADC therapy in a real-world setting.
Methods
This retrospective analysis includes all patients who were treated with either T-DXd or SG or both outside of clinical trials at the Department of Gynecology and Obstetrics, Ulm University Hospital, Germany, at the Department of Women’s Health at Tuebingen University Hospital, Germany and at the Department of Gynecology and Obstetrics, Freiburg University, Germany between January 01, 2018 and June 30, 2023. The data cut-off date of August 31, 2023 was used for data analysis. The study was conducted in compliance with the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of Tuebingen University (380/2020BO), Freiburg University (23–1506-S1-AV) and Ulm University (158/23). SG was administered at a dose of 10 mg/kg of body weight on days one and eight within a 21-day cycle, continuing until disease progression or the onset of intolerable toxicities, in accordance with clinical standards. T-DXd was administered at a dose of 5.4 mg/kg of body weight on the first day of a 21-day cycle until disease progression or the onset of intolerable toxicities, in line with clinical standards. Dose reductions were carried out in accordance with prescribing information. In 9 out of 48 patients (18.8%) treated with SG, therapy was initiated at a reduced dosage of 7.5 mg/kg of body weight. In 12 out of 78 patients (15.4%) treated with T-DXd, therapy was initiated at a reduced dosage of either 4.4 mg/kg (7 patients) or 3.2 mg/kg (5 patients). Reasons for initiating therapy at a reduced dose included general condition, multiple prior therapies, comorbidities, or laboratory abnormalities. Details regarding the treatment regimen and concomitant medication can be found in the appendix (Suppl. Tables S4 and S5 ). Treatment emergent adverse events (TEAEs) were assessed using the Common Terminology Criteria for Adverse Events (CTCAE V5.0), with this analysis focusing solely on hematotoxicity and interstitial lung disease (ILD). Additional side effects were excluded from this analysis, as only hematotoxicity and ILD were systematically documented across all three centers. In the context of discontinuation and dose reductions due to TEAEs, all adverse events were considered, as they were consistently documented in these cases.
In accordance with local, clinical practice, chemotherapy efficacy was assessed by full-body CT scan every 8–12 weeks. All patients with known brain metastases underwent baseline magnetic resonance imaging (MRI) of the brain, and follow-up MRIs were conducted at least every three months, according to local standards, as described previously 22 . Hormone receptor (HR) and HER2 receptor expression were assessed by board-certified pathologists, according to local standards, as previously described 23 24 25 , on the most recent available biopsy prior to the indication for ADC therapy. If no biopsy of the metastasis was available, the indication for therapy and subtype classification were based on the most recent available biopsy, in accordance with clinical routine. Real-world, progression-free survival (rwPFS) was defined as the duration from treatment initiation to disease progression or death, whichever occurred first. Patients lost to follow-up were censored at their last-recorded contact date. Real-world, overall survival (rwOS) was defined as the duration from the start of T-DXd or SG treatment to death. Patients without an OS event were censored at the last-known time they were alive. The Kaplan–Meier method was used to estimate the distribution of rwPFS and rwOS. Median follow-up was calculated using the reverse Kaplan–Meier method 26 . Data processing, statistical analysis and visualization were conducted, as described previously 23 24 .
Results
Sacituzumab govitecan
Forty-eight patients with mBC who were treated with SG were included in the study, with 43 having TNBC and 5 having HR+/HER2− subtype ( Table 1 ). The median age at the initiation of SG therapy was 55.5 years (range: 27–82 years). The median therapy duration was 3.5 months (range: 0.5–15.3 months), varying between TNBC (3.7 months; range: 0.5–15.3 months) and HR+/HER2− (2.6 months; range: 0.5–7.4 months). Before SG therapy, patients had undergone a mean of 3.7 (standard deviation (SD) ± 2.3 therapies) systemic therapies in the metastatic setting, differing between TNBC (3.3 therapies; SD ± 1.8 therapies) and HR+/HER2− (7.4 therapies; SD ± 2.7 therapies) subgroups. Further patients’ characteristics are reported in Table 1 .
Table 1 Characteristics of patients treated with sacituzumab govitecan.
| SG total | TNBC | HR+/HER2− | |
| adj. = adjusted; BRA mets = brain metastases; DR = dose reduction; ER = estrogen receptor; HER2 = human epidermal growth factor receptor 2; ILC = invasive lobular carcinoma; n/a = not available; NE = not estimable; NR = not reached; NST = non-special type; PR = progesterone receptor; SD = standard deviation; TNBC = triple-negative breast cancer. Mean therapy line: Includes all therapy lines from the neo(adjuvant) and metastatic settings. Mean therapy line M1: Only counts therapy lines administered in the metastatic setting. | |||
| n (%) | 48 (100) | 43 (89.6) | 5 (10.4) |
| Median age in years (range) | 55.5 (27–82) | 55.0 (27–82) | 56.0 (55–60) |
| Histology | |||
|
45 (93.8) | 41 (95.3) | 4 (80) |
|
2 (4.2) | 1 (2.3) | 1 (20) |
|
1 (2.1) | 1 (2.3) | 0 (0.0) |
| De novo metastatic disease (%) | 11 (22.9) | 11 (25.6) | 0 (0.0) |
| Mean metastatic sites ± SD | 2.4 ± 1.2 | 2.4 ± 1.2 | 2.2 ± 1.1 |
| BRA mets – no. of patients (%) | 12 (25.0) | 12 (27.9) | 0 (0.0) |
| Mean therapy line ± SD | 4.9 ± 2.4 | 4.4 ± 2.0 | 8.8 ± 2.2 |
| Mean therapy line M1 ± SD | 3.7 ± 2.3 | 3.3 ± 1.8 | 7.4 ± 2.7 |
|
19 (39.6) | 14 (32.6) | 5 (100) |
| Median therapy duration in months (range) | 3.5 (0.5–15.3) | 3.4 (0.5–15.3) | 2.6 (0.5–7.4) |
| Mean Dose density (% full dose) ± SD | 78.8 ± 18.6 | 77.9 ± 18.8 | 86.8 ± 16.1 |
| Mean Dose density (% adj. for DR) ± SD | 89.6 ± 13.0 | 89.7 ± 13.2 | 88.9 ± 11.9 |
| Median follow-up in months (95% CI) | 11.8 (8.0–14.5) | 12.5 (8.9–15.2) | 7.2 (2.6–8.0) |
| Median rwPFS in months (95% CI) | 4.9 (2.8–6.3) | 4.9 (2.8–6.3) | NR (NE) |
| Median rwOS in months (95% CI) | 12.4 (8.7–NE) | 12.4 (6.7–NE) | NR (3.2–NE) |
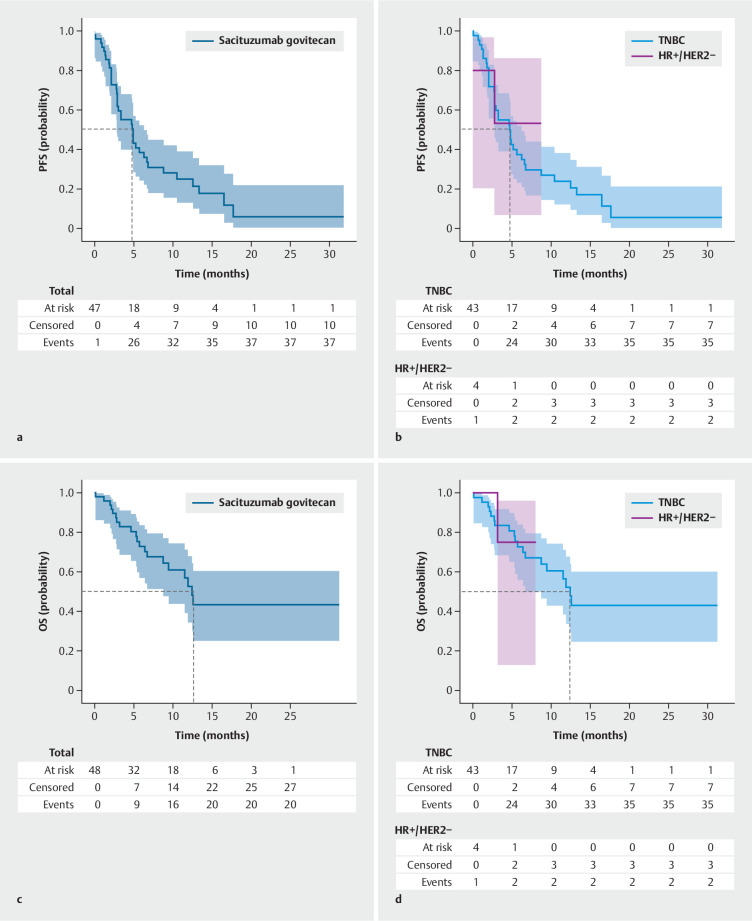
Having a median follow-up of 12.5 months (95% CI: 8.9–15.2) in the TNBC subgroup, the overall median rwPFS was 4.9 months (95% CI: 2.8–6.3; Fig. 1 ). The median OS in the TNBC real-world population was 12.4 months (95% CI, 6.7–NE; Fig. 1 ). For the HR+/HER2− subgroup, rwPFS and rwOS were not reached after a mean follow-up of 7.2 months (95% CI: 2.6–8.0).
Fig. 1.
Real-world progression free survival and real-world overall survival with sacituzumab govitecan: The study cohort consisted of a total of 48 patients, comprising 43 patients with TNBC and 5 patients with HR+/HER2− mBC. Panel a illustrates the rwPFS of all patients treated with sacituzumab govitecan. Panel b illustrates the rwPFS for patients diagnosed with triple-negative mBC and HR+/HER2− mBC treated with sacituzumab govitecan separately. Panel c illustrates the rwOS of all patients treated with sacituzumab govitecan. Panel d illustrates the rwOS of patients diagnosed with triple-negative mBC and HR+/HER2− mBC treated with sacituzumab govitecan separately.
As of the data cut-off, SG was still being administered to 11 out of 48 patients (23%; Suppl. Table S1 ). Of the 48 patients, 37 discontinued treatment (77.1%): 26 due to progression (54.2 %), two due to adverse events (4.2%), three due to patient preference (6.3%) and four due to death (8.3%). Overall, 20 out of 48 patients treated with SG had died by the data cut-off date (41.7%; Suppl. Table S1 ).
Patients received an average of 78.8 % of the intended full dose according to the protocol throughout their therapy duration, which increased to 89.6 % when accounting for dose reductions implemented during treatment. In 15 out of 48 patients (31.3%), at least one dose reduction was carried out. In 9 out of 48 patients (18.8%), therapy was initiated at a reduced dosage, due to general condition, multiple, prior therapies, comorbidities or laboratory abnormalities. Neutropenia (11/15) was the primary reason for a dose reduction. Two patients (4.2%) discontinued SG therapy due to TEAEs, both of which were prolonged, severe neutropenia. Three patients discontinued SG therapy at their own, unspecified request. TEAEs are reported in Table 2 . Severe neutropenia (grade 3 or higher) was the predominant side effect, occurring in 33.3% of patients. The use of primary prophylactic granulocyte colony stimulating factor (G-CSF) was associated with a lower incidence of severe neutropenia (24.1% vs. 47.4%; non-significant: p = 0.09).
Table 2 Adverse events in SG therapy: hematotoxicity and interstitial lung disease (ILD) were retrospectively assessed as adverse events. Grading was performed according to the Common Terminology Criteria for Adverse Events. G-CSF denotes granulocyte colony-stimulating factor.
| Adverse event | n | % |
| *: Dose reductions due to treatment-associated adverse events of all types. × : Therapy discontinuation due to treatment-associated adverse events of all types or at the patient’s request. | ||
| Neutropenia all grades (%) | 27 | 56.3 |
|
16 | 33.3 |
|
7/22 | 24.1 |
|
9/19 | 47.4 |
| Anemia all grades (%) | 19 | 39.6 |
|
3 | 6.3 |
| Thrombocytopenia all grades | 6 | 12.5 |
|
0 | 0.0 |
| ILD all grades | 2 | 4.2 |
|
0 | 0.0 |
| Dose reduction* | 15 | 31.3 |
| Discontinuation of therapy × | 5 | 10.4 |
Trastuzumab deruxtecan
A total of 77 patients diagnosed with mBC were treated with T-DXd, with 41 classified as HER2+ and 36 as HER2-low ( Table 3 ). The median age at therapy initiation was 60.0 years (range: 36–83 years). The median duration of T-DXd therapy was 6.0 months (range: 0.7–22.1 months), with a longer duration observed in the HER2+ subgroup (8.3 months; (range: 0.7–22.1 months), compared to the HER2-low subgroup (4.8 months; range: 0.7–14.7 months). Prior to receiving T-DXd, patients had undergone a mean of 4.2 (SD ± 2.5 therapies) systemic therapies in the metastatic setting (HER2+: 3.7 therapies; SD ± 2.6 therapies; HER2-low: 4.7 therapies; SD ± 2.2 therapies).
Table 3 Characteristics of patients treated with T-DXd.
| T-DXd total | HER2+ | HER2-low | |
| adj. = adjusted; BRA mets = brain metastases; DR = dose reduction; ER = estrogen receptor; HER2 = human epidermal growth factor receptor 2; ILC = invasive lobular carcinoma; n/a = not available; NE = not estimable; NR = not reached; NST = non-special type; PR = progesterone receptor; SD = standard deviation; TNBC = triple-negative breast cancer. Mean therapy line: Includes all therapy lines from the neo(adjuvant) and metastatic settings. Mean therapy line M1: Only counts therapy lines administered in the metastatic setting. | |||
| n (%) | 77 (100) | 41 (53.2) | 36 (46.8) |
| Median Age (range) | 60.0 (36–83) | 58.0 (36–83) | 61.0 (36–79) |
| Histology | |||
|
63 (81.8) | 33 (80.5) | 30 (83.3) |
|
8 (10.4) | 4 (9.8) | 4 (11.1) |
|
4 (5.2) | 2 (4.9) | 2 (5.6) |
|
2 (2.6) | 1 (2.4) | 1 (2.7) |
| Hormone receptor status (HR) | |||
|
44 (57.1) | 18 (43.9) | 26 (72.2) |
|
33 (42.9) | 23 (56.1) | 10 (27.8) |
|
1 (0.01) | 1 (0.02) | 0 (0.0) |
| De novo metastatic disease (%) | 26 (33.8) | 16 (39.0) | 10 (27.8) |
| Mean metastatic sites ± SD | 2.5 ± 1.2 | 2.5 ± 1.2 | 2.5 ± 1.2 |
| BRA mets – no. of patients (%) | 16 (20.8) | 11 (26.8) | 5 (13.9) |
| Mean therapy line ± SD | 5.3 ± 2.6 | 4.8 ± 2.7 | 5.9 ± 2.5 |
| Median therapy line (range) | 4.0 (2–14) | 4.0 (2–12) | 4.0 (3–14) |
| Mean therapy line M1 ± SD | 4.2 ± 2.5 | 3.7 ± 2.6 | 4.7 ± 2.2 |
| Median therapy line M1 (range) | 3 (1–12) | 3 (1–12) | 3 (2–10) |
|
59 (76.6) | 2 (65.9) | 32 (88.9) |
|
37 (48.1) | 14 (34.1) | 22 (61.1) |
|
6.0 (0.7–22.1) | 8.3 (0.7–22.1) | 4.8 (0.7–14.7) |
| Mean dose density (% full dose) ± SD | 87.6 ± 15.2 | 86.9 ± 16.2 | 92.4 ± 12.4 |
| Mean dose density (% adj. for DR) ± SD | 95.7 ± 7.7 | 94.0 ± 9.3 | 98.4 ± 5.0 |
| Median follow-up in months (95% CI) | 10.4 (8.4–11.6) | 14.0 (8.7–20.1) | 7.6 (6.4–11.2) |
| Median rwPFS in months (95% CI) | 8.6 (5.8–12.4) | 10.8 (7.7–15.8) | 4.7 (3.1–10.8) |
| Median rwOS in months (95% CI) | 23.8 (16.1–NE) | 27.1 (16.1–NE) | NR (7.8–NE) |
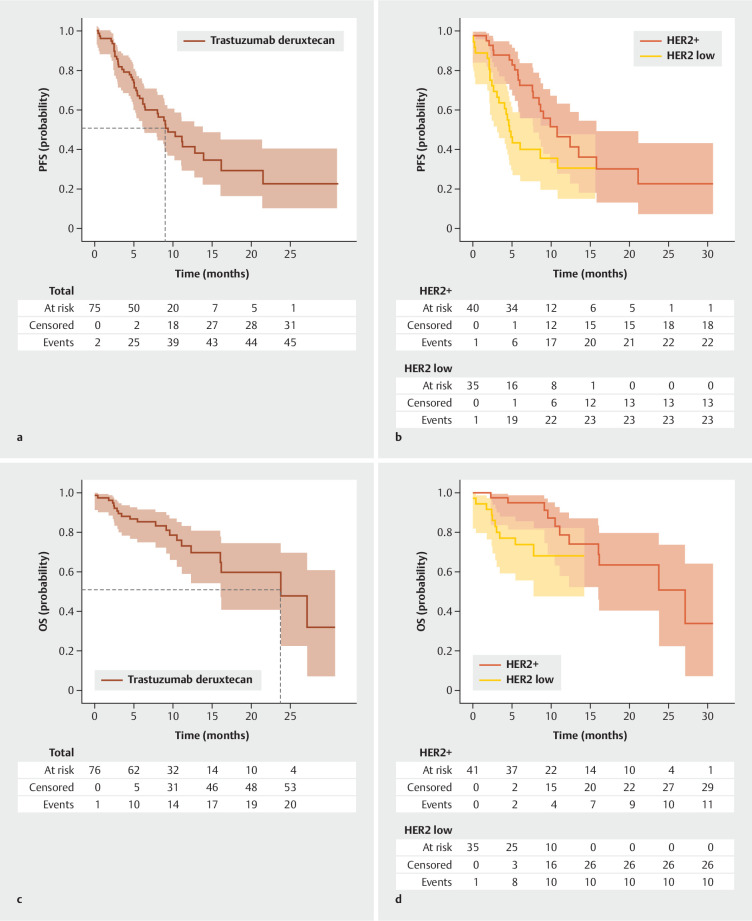
At a median follow-up of 14.0 months (95% CI: 8.7–20.1 months), the HER2+ subgroup showed a median rwPFS of 10.8 months (95% CI: 7.7–15.8 months; Fig. 2 ) and a median rwOS of 27.1 months (95% CI: 16.1–NE months; Fig. 2 ). In the HER-low subgroup, at a median follow-up of 7.6 months (95% CI: 6.4–11.2 months), the median rwPFS was 4.7 months (95% CI: 3.1–10.8 months; Fig. 2 ), while the median rwOS was not reached ( Fig. 2 ). As of the data cut-off, T-DXd was still being administered to 32 out of 77 patients (42%; Suppl. Table S2 ). Of the 77 patients, 45 discontinued treatment (58.4%): 30 due to progression (39.0 %), 10 due to adverse events (13.0%), two due to patient’s request (2.6%), two due to death (2.6%) and one due to unknown reason (1.3%). Overall, 22 out of 77 patients treated with T-DXd had died by the data cut-off date (28.6%; Suppl. Table S2 ).
Fig. 2.
Real-world progression free survival with trastuzumab deruxtecan: The study cohort consisted of a total of 77 patients, comprising 41 patients with HER2+ mBC and 36 patients with HER2-low mBC. Panel a illustrates the rwPFS of all patients treated with T-DXd. Panel b illustrates the rwPFS for patients diagnosed with HER2+ and HER2-low mBC treated with T-DXd separately. Panel c illustrates the rwOS of all patients treated with T-DXd. Panel d illustrates the rwOS for patients diagnosed with HER2+ and HER2-low mBC treated with T-DXd separately.
Patients received an average of 87.6 % of the intended full dose according to the protocol throughout their therapy duration, which increased to 95.7 % when accounting for dose reductions implemented during treatment. In 12 out of 77 (15.6%) patients (HER2+: 7; HER2-low: 5), therapy was initiated at a reduced dosage due to general condition, multiple prior therapies, comorbidities, or laboratory abnormalities. In 13 out of 77 patients (16.9%), at least one dose reduction was carried out during the course of therapy. Table 4 displays the recorded TEAEs of T-DXd. Therapy-associated interstitial lung disease (ILD) occurred in eight patients (10.4%), and, among them, 2 (2.6%) experienced ILD at grade 3. There were no cases of ILD higher than Grade 3. The median time to onset of ILD was 5.0 months (7 days – 15.7 months). TEAEs led to the discontinuation of treatment in 10 patients (13.0%). The most common reason for treatment discontinuation was the occurrence of ILD (7/10), followed by fatigue (3/10). Two patients discontinued T-DXd therapy at their own, unspecified request.
Table 4 Adverse events in T-DXd therapy: Hematotoxicity and interstitial lung disease (ILD) were retrospectively assessed as adverse events. Grading was performed according to the Common Terminology Criteria for Adverse Events. G-CSF denotes Granulocyte colony-stimulating factor.
| Adverse event | n | % |
| *: Dose reductions due to treatment-associated adverse events of all types. × : Therapy discontinuation due to treatment-associated adverse events of all types or at the patient’s request. | ||
| Neutropenia all grades | 34 | 44.2 |
|
6 | 7.8 |
| Anemia all grades | 19 | 24.7 |
|
3 | 3.9 |
| Thrombocytopenia all grades | 9 | 11.7 |
|
2 | 2.6 |
| ILD all grades | 8 | 10.4 |
|
2 | 2.6 |
| Dose reduction* | 13 | 16.9 |
| Discontinuation of therapy × | 12 | 15.6 |
Discussion
This retrospective study aims to evaluate the feasibility, tolerability and efficacy of third-generation ADCs SG and T-DXd in real-world settings. Our cohort includes pre-treated patients, many of whom do not meet phase III trial criteria, offering valuable insights into clinical practice. When assessing therapy feasibility and compliance, we opted for an indirect yet informative approach by comparing the actual administered dose over the entire therapy duration to the theoretical dose specified by the protocol. Taking account of the dose reductions that occurred, 89.6% of the prescribed SG dose, and 95.7% of the prescribed T-DXd dose were administered, indicating that third generation ADCs could be administered without significant interruptions.
Regarding efficacy, this study robustly confirms the positive outcomes observed in clinical trials and existing monocentric real-world evidence (RWE) for SG treatment in mTNBC 6 11 27 . However, some notable differences should be highlighted. In the ASCENT study, 12% of patients had brain metastases, compared to 23% in a real-world analysis, presented by Reinisch et al. and 25% in our cohort 6 27 . We recently reported on the effectiveness of SG and T-DXd in patients with both stable and active brain metastasis within this cohort 22 . Regarding prior therapy, there are differences between the three studies in the median line of treatment in the metastatic setting of three in our cohort, compared to two in the existing RWE by Reinisch et al. and three in the ASCENT trial 6 27 . A subgroup analysis of the ASCENT trial, which included only second-line patients, demonstrated a median PFS of 5.7 months and a median OS of 10.9 months 28 . Our analysis demonstrated that the positive effect on rwPFS remains stable even when given in later therapy lines. (Suppl. Fig. S1 ). In summary, this real-world analysis confirms the consistent efficacy of SG therapy for mTNBC.
For T-DXd therapy, we observed lower efficacy as compared to clinical trial data and previous real-world evidence 5 18 20 29 , especially in the HER2-positive cohort, which might be attributed to several factors. Firstly, the outcome data for T-DXd therapy in our analysis are not yet mature, with a significant proportion of patients still receiving T-DXd therapy (42%) at data cut-off (Suppl. Table S2 ). Secondly, the patients in our real-world cohort were older and therefore presumably had more comorbidities, which is also reflected in the relevant proportion of patients for whom treatment was started with a reduced dose. Thirdly, as compared to the Destiny-Breast trials, a substantially higher proportion had brain metastases including patients with active brain metastases who would not have been eligible for participation in those trials, with 26.8% in the HER2-positive subgroup and 13.9% in the HER2-low subgroup as compared to 16% and 5.4% respectively 5 20 . Given the lack of routine screening for brain metastases in real-world settings, the true prevalence in this cohort might even be underestimated 30 . Finally, patients in our current analysis had more prior treatments, with 65.9% of HER2+ and 88.9% of HER2-low mBC patients having received ≥ 3 previous therapy lines for metastatic disease, compared to 27.9% and 62,7% in DB04 and DB03 respectively 5 20 , with potential impact on the outcome (Suppl. Fig. S2 ). It should also be noted that nearly one-sixth of patients started T-DXd therapy at a lower dose. Not only would these patients have been excluded from the corresponding clinical trials but it also reflects the clinical reality where T-DXd is frequently given as a final therapy option later in the disease progression. Additionally, differences were observed with regard to the proportion of HR+ patients (Suppl. Table S3 ). However, consistent with previous literature, no difference regarding efficacy was observed, based on HR-status (Suppl. Fig. S3 ) 4 5 . With respect to HER2-low mBC, this analysis represents the first set of available RWE to our knowledge. For HER2+ mBC, the Italian DE-REAL study showed a longer rwPFS of 16 months (95% CI: 13–19) but shorter rwOS of 20 months (95% CI: 19–31) than our RWE 29 . Comparable to our analysis, patients had a median age of 66 years, with 25% having brain metastases and 25% being HR-negative 29 .
Neutropenia was the most frequent adverse event observed with SG therapy, with ≥ Grade 3 neutropenia occurring in 33.3% of patients. This is in line with existing RWE by Reinisch et al., with 27.9% experiencing ≥ Grade 3 neutropenia 27 but less frequent than in clinical trials (IMMU-132: 42.4%, ASCENT: 51.2%) 10 11 . Possible contributing factors might include under-reporting in retrospective RWE and greater use of G-CSF. Although not statistically significant, our analysis observed a numerically reduced rate of higher-grade neutropenia with primary prophylactic G-CSF use. The ongoing phase II PRIMED trial (NCT05520723) is examining the impact of G-CSF and other co-medications on SG therapy’s safety profile.
T-DXd therapy exhibited hematotoxicity similar to that observed in clinical trials 5 20 . The rate of therapy-associated ILD was also in line with data from clinical trial data and existing RWE 3 5 29 31 . Unlike trials, no cases of ILD > grade 3 or ILD-associated deaths were observed in our real-world cohort, likely due to improved clinical standards in managing this side effect 32 .
The limitations of our analysis include a small sample size, a heterogeneous patient population and a relatively short follow-up. Due to the retrospective character of our analysis, assessments of PFS, OS and adverse event monitoring in this study lacked standardization and relied on routine, clinical evaluation. Reporting of additional side effects, apart from hematotoxicity and ILD, which were systematically documented in all three centers, was deliberately omitted for this reason. Nonetheless, the multicenter design of this study is a strength of this retrospective, real-world analysis, given the limited data available on the efficacy of ADCs in real-world settings.
Conclusion
Our study presents, multicentric, real-world efficacy data for SG therapy in mTNBC, which closely aligns with the safety profile and efficacy outcomes observed in previous clinical trials. In line with existing, monocentric, RWE, therapy with SG is feasible, effective and does not raise new safety concerns in the clinical setting. Since neutropenia is the most common severe AE with SG therapy, risk-reducing primary prophylactic use of GCSF is recommended, based on our data and clinical experience. T-DXd therapy demonstrated efficacy in both HER2+ and HER2-low subgroups, albeit slightly lower than in clinical trials. However, longer follow-up is needed for definitive conclusions in real-world settings.
Funding: This research received no external funding.
Ethical Approval: The study was conducted in compliance with the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of Tuebingen University (380/2020BO), Freiburg University (23–1506-S1-AV) and Ulm University (158/23).
Declaration of generative AI and AI-assisted technologies in the writing process: During the preparation of this work the authors used ChatGPT in order to improve language and readability. After using this tool the authors reviewed and edited the content as well as the spelling as needed and take full responsibility for the content of the publication.
Supplementary Material
Suppl. Table S1 : Distribution of Patients with SG Therapy at Data Cutoff.
Suppl. Table S2 : Distribution of Patients with T-DXd Therapy at Data Cutoff.
Suppl. Table S3 : Real-world progression-free survival and real-world overall survival with T-DXd therapy stratified by hormone receptor status.
Suppl. Table S4
Suppl. Table S5
Suppl. Fig. S1 : Real-world progression-free survival with SG therapy stratified by therapy line: Panel A illustrates the rwPFS of all patients treated with SG segregated into those who received two or fewer prior therapies in the metastatic setting and those who received more than two prior therapies in the metastatic setting. Panel B illustrates the rwPFS of all patients treated with SG segregated into those who received three or fewer prior therapies in the metastatic setting and those who received more than three prior therapies in the metastatic setting.
Suppl. Fig. S2 : Real-world progression-free survival with T-DXd therapy stratified by therapy line: Panel A illustrates the rwPFS of all patients with HER2-low mBC treated with T-DXd segregated into those who received two or fewer prior therapies in the metastatic setting and those who received more than two prior therapies in the metastatic setting. Panel B illustrates the rwPFS of all patients with HER2-low mBC treated with T-DXd segregated into those who received three or fewer prior therapies in the metastatic setting and those who received more than three prior therapies in the metastatic setting. Panel C depicts the real-world PFS of all patients with HER2+ mBC treated with T-DXd up to the second therapy line versus beyond the second therapy line in the metastatic setting. Panel D depicts the real-world PFS of all patients with HER2+ mBC treated with T-DXd up to the third therapy line versus beyond the third therapy line in the metastatic setting.
Suppl. Fig. S3 : Real-world progression-free survival and real-world overall-survival with T-DXd therapy stratified by hormone receptor status.
Acknowledgement
We would like to thank Mrs. Seitz for the linguistic and grammatical revision of the manuscript.
Footnotes
Conflict of Interest H. Schäffler: Travel Support by Daiichi Sankyo and Gilead, Honoraria by Novartis; D. Jakob: none; S. Huesmann: none; K. Pfister: Honoraria by Pfizer Novartis and Gilead; K. Veselinovic: Honoraria by Roche, Pfizer, AstraZeneca; F. Schochter: Travel Support, Honoraria and Consulting Fees by AstraZeneca, Roche, Karyopharm, Merck, GlaxoSmithKline, Eisai, Clovis; E. Leinert: none; V. Fink: none; B. Rack: Honoraria and research funding by AstraZeneca and Novartis; A. Englisch: none; L. Volmer: none; T. Engler: Travel Support, Honoraria and Consulting Fees by AstraZeneca, GSK, Gilead, Novartis, Lilly, Pfizer, MSD, Pierre Fabre, Stemline, Roche, Daiichi Sankyo; ML. Frevert: Honoraria by Novartis; I. Juhasz-Böss: none; S. Brucker: Travel Support and Consulting Fees by Hologic, Sanofi, AstraZeneca, Lilly, MSD, Medtronic, Pfizer; S. Heublein: research funding and honoraria from Clovis Oncology, Inc., GlaxoSmithKline, Novartis, Roche, AstraZeneca and Pfizer. Participation in advisory boards for Novartis, GlaxoSmithKline, and Merck Sharp & Dohme Corporation; W. Janni: Travel Support, Honoraria and Consulting Fees by Daiichi Sankyo, AstraZeneca, Gilead; FA. Taran: Travel Support by Gilead, Consulting Fees by AstraZeneca, Gilead, MSD, ImmunoGen, Onkowissen, Roche, GSK; A. Hartkopf: Travel Support by Daiichi Sankyo, Gilead, Roche, AstraZeneca, Pfizer, Honoraria by Daiichi Sankyo, Gilead, Seagen, Roche, AstraZeneca, Pfizer, Lilly, MSD, Novartis; D. Dannehl: Travel Support by Gilead and Daiichi Sankyo, Honoraria by Gilead.
Supplementary Material
References
- 1.Arnold M, Morgan E, Rumgay H et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast. 2022;66:15–23. doi: 10.1016/j.breast.2022.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams E, Wildiers H, Neven P et al. Sacituzumab govitecan and trastuzumab deruxtecan: two new antibody–drug conjugates in the breast cancer treatment landscape. ESMO Open. 2021;6:100204. doi: 10.1016/J.ESMOOP.2021.100204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hurvitz SA, Hegg R, Chung WP et al. Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet. 2023;401:105–117. doi: 10.1016/S0140-6736(22)02420-5. [DOI] [PubMed] [Google Scholar]
- 4.Modi S, Saura C, Yamashita T et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N Engl J Med. 2020;382:610–621. doi: 10.1056/NEJMoa1914510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Modi S, Jacot W, Yamashita T et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N Engl J Med. 2022;387:9–20. doi: 10.1056/NEJMOA2203690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bardia A, Hurvitz SA, Tolaney SM et al. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. N Engl J Med. 2021;384:1529–1541. doi: 10.1056/NEJMOA2028485. [DOI] [PubMed] [Google Scholar]
- 7.Starodub AN, Ocean AJ, Shah MA et al. First-in-Human Trial of a Novel Anti-Trop-2 Antibody-SN-38 Conjugate, Sacituzumab Govitecan, for the Treatment of Diverse Metastatic Solid Tumors. Clin Cancer Res. 2015;21:3870–3878. doi: 10.1158/1078-0432.CCR-14-3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharkey RM, McBride WJ, Cardillo TM et al. Enhanced Delivery of SN-38 to Human Tumor Xenografts with an Anti-Trop-2-SN-38 Antibody Conjugate (Sacituzumab Govitecan) Clin Cancer Res. 2015;21:5131–5138. doi: 10.1158/1078-0432.CCR-15-0670. [DOI] [PubMed] [Google Scholar]
- 9.Bardia A, Mayer IA, Vahdat LT et al. Sacituzumab Govitecan-hziy in Refractory Metastatic Triple-Negative Breast Cancer. N Engl J Med. 2019;380:741–751. doi: 10.1056/NEJMOA1814213. [DOI] [PubMed] [Google Scholar]
- 10.Bardia A, Messersmith WA, Kio EA et al. Sacituzumab govitecan, a Trop-2-directed antibody-drug conjugate, for patients with epithelial cancer: final safety and efficacy results from the phase I/II IMMU-132–01 basket trial. Ann Oncol. 2021;32:746–756. doi: 10.1016/J.ANNONC.2021.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Bardia A, Rugo HS, Tolaney SM et al. Final Results From the Randomized Phase III ASCENT Clinical Trial in Metastatic Triple-Negative Breast Cancer and Association of Outcomes by Human Epidermal Growth Factor Receptor 2 and Trophoblast Cell Surface Antigen 2 Expression. J Clin Oncol. 2024;42:1738–1744. doi: 10.1200/JCO.23.01409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.European Medicines Agency . Trodelvy. https://www.ema.europa.eu/en/medicines/human/EPAR/trodelvy https://www.ema.europa.eu/en/medicines/human/EPAR/trodelvy
- 13.U.S. Food & Drug Administration ; (FDA) . FDA grants regular approval to sacituzumab govitecan for triple-negative breast cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-regular-approval-sacituzumab-govitecan-triple-negative-breast-cancer https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-regular-approval-sacituzumab-govitecan-triple-negative-breast-cancer
- 14.Kalinsky K, Diamond JR, Vahdat LT et al. Sacituzumab govitecan in previously treated hormone receptor-positive/HER2-negative metastatic breast cancer: final results from a phase I/II, single-arm, basket trial. Ann Oncol. 2020;31:1709–1718. doi: 10.1016/J.ANNONC.2020.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Rugo HS, Bardia A, Marmé F et al. Sacituzumab Govitecan in Hormone Receptor-Positive/Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer. J Clin Oncol. 2022;40:3365–3376. doi: 10.1200/JCO.22.01002. [DOI] [PubMed] [Google Scholar]
- 16.Rugo HS, Bardia A, Marmé F et al. Overall survival with sacituzumab govitecan in hormone receptor-positive and human epidermal growth factor receptor 2-negative metastatic breast cancer (TROPiCS-02): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2023;402:1423–1433. doi: 10.1016/S0140-6736(23)01245-X. [DOI] [PubMed] [Google Scholar]
- 17.Nakada T, Sugihara K, Jikoh T et al. The Latest Research and Development into the Antibody-Drug Conjugate, [fam-] Trastuzumab Deruxtecan (DS-8201a), for HER2 Cancer Therapy. Chem Pharm Bull (Tokyo) 2019;67:173–185. doi: 10.1248/CPB.C18-00744. [DOI] [PubMed] [Google Scholar]
- 18.Modi S, Saura C, Yamashita T et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N Engl J Med. 2020;382:610–621. doi: 10.1056/NEJMOA1914510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Modi S, Saura C, Yamashita T et al. Abstract PD3–06: Updated results from DESTINY-breast01, a phase 2 trial of trastuzumab deruxtecan (T-DXd) in HER2 positive metastatic breast cancer. Cancer Res. 2021;81:PD3–06. doi: 10.1158/1538-7445.SABCS20-PD3-06. [DOI] [Google Scholar]
- 20.Cortés J, Kim S-B, Chung W-P et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine for Breast Cancer. N Engl J Med. 2022;386:1143–1154. doi: 10.1056/NEJMOA2115022. [DOI] [PubMed] [Google Scholar]
- 21.Ogitani Y, Aida T, Hagihara K et al. DS-8201a, A Novel HER2-Targeting ADC with a Novel DNA Topoisomerase I Inhibitor, Demonstrates a Promising Antitumor Efficacy with Differentiation from T-DM1. Clin Cancer Res. 2016;22:5097–5108. doi: 10.1158/1078-0432.CCR-15-2822. [DOI] [PubMed] [Google Scholar]
- 22.Dannehl D, Jakob D, Mergel F et al. The efficacy of sacituzumab govitecan and trastuzumab deruxtecan on stable and active brain metastases in metastatic breast cancer patients—a multicenter real-world analysis. ESMO Open. 2024;9:102995. doi: 10.1016/j.esmoop.2024.102995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schäffler H, Mergel F, Pfister K et al. The Clinical Relevance of the NATALEE Study: Application of the NATALEE Criteria to a Real-World Cohort from Two Large German Breast Cancer Centers. Int J Mol Sci. 2023;24:16366. doi: 10.3390/IJMS242216366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dannehl D, Engler T, Volmer LL et al. Which Patients Do We Need to Test for BRCA1/2 Mutation? Feasibility of Adjuvant Olaparib Treatment in Early Breast Cancer–Real-World Data from Two Large German Breast Centers. Cancers. 2023;15:3847. doi: 10.3390/CANCERS15153847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dannehl D, Volmer LL, Weiss M et al. Feasibility of Adjuvant Treatment with Abemaciclib-Real-World Data from a Large German Breast Center. J Pers Med. 2022;12:382. doi: 10.3390/JPM12030382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shuster JJ. Median follow-up in clinical trials. J Clin Oncol. 1991;9:191–192. doi: 10.1200/JCO.1991.9.1.191. [DOI] [PubMed] [Google Scholar]
- 27.Reinisch M, Bruzas S, Spoenlein J et al. Safety and effectiveness of sacituzumab govitecan in patients with metastatic triple-negative breast cancer in real-world settings: first observations from an interdisciplinary breast cancer centre in Germany. Ther Adv Med Oncol. 2023;15 doi: 10.1177/17588359231200454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carey LA, Loirat D, Punie K et al. Sacituzumab govitecan as second-line treatment for metastatic triple-negative breast cancer-phase 3 ASCENT study subanalysis. NPJ Breast Cancer. 2022;8:72. doi: 10.1038/S41523-022-00439-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Botticelli A, Caputo R, Scagnoli S et al. Real-World Outcomes of Trastuzumab Deruxtecan in Patients With HER2+ Metastatic Breast Cancer: The DE-REAL Study. Oncologist. 2024;29:303–310. doi: 10.1093/ONCOLO/OYAD308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cardoso F, Senkus E, Costa A et al. 4th ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 4)†. Ann Oncol. 2018;29:1634–1657. doi: 10.1093/ANNONC/MDY192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Powell CA, Modi S, Iwata H et al. Pooled analysis of drug-related interstitial lung disease and/or pneumonitis in nine trastuzumab deruxtecan monotherapy studies. ESMO Open. 2022;7:100554. doi: 10.1016/J.ESMOOP.2022.100554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rugo HS, Bianchini G, Cortes J et al. Optimizing treatment management of trastuzumab deruxtecan in clinical practice of breast cancer. ESMO Open. 2022;7:100553. doi: 10.1016/J.ESMOOP.2022.100553. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.