Abstract
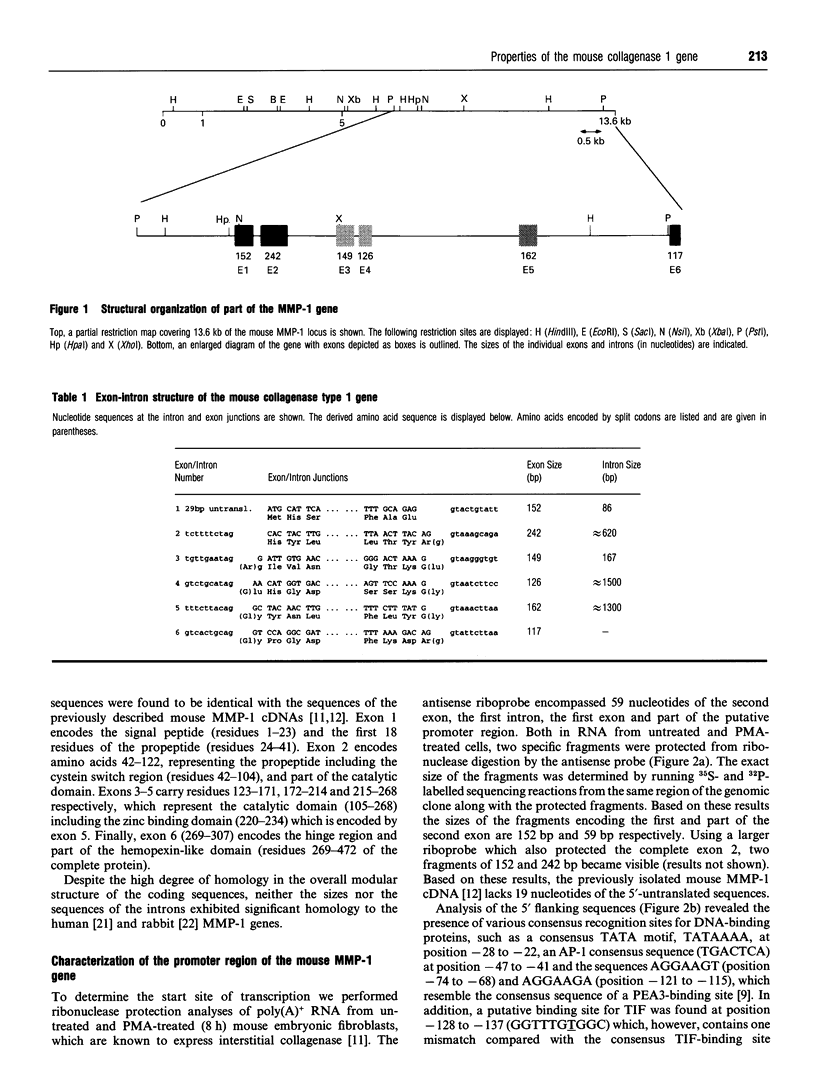
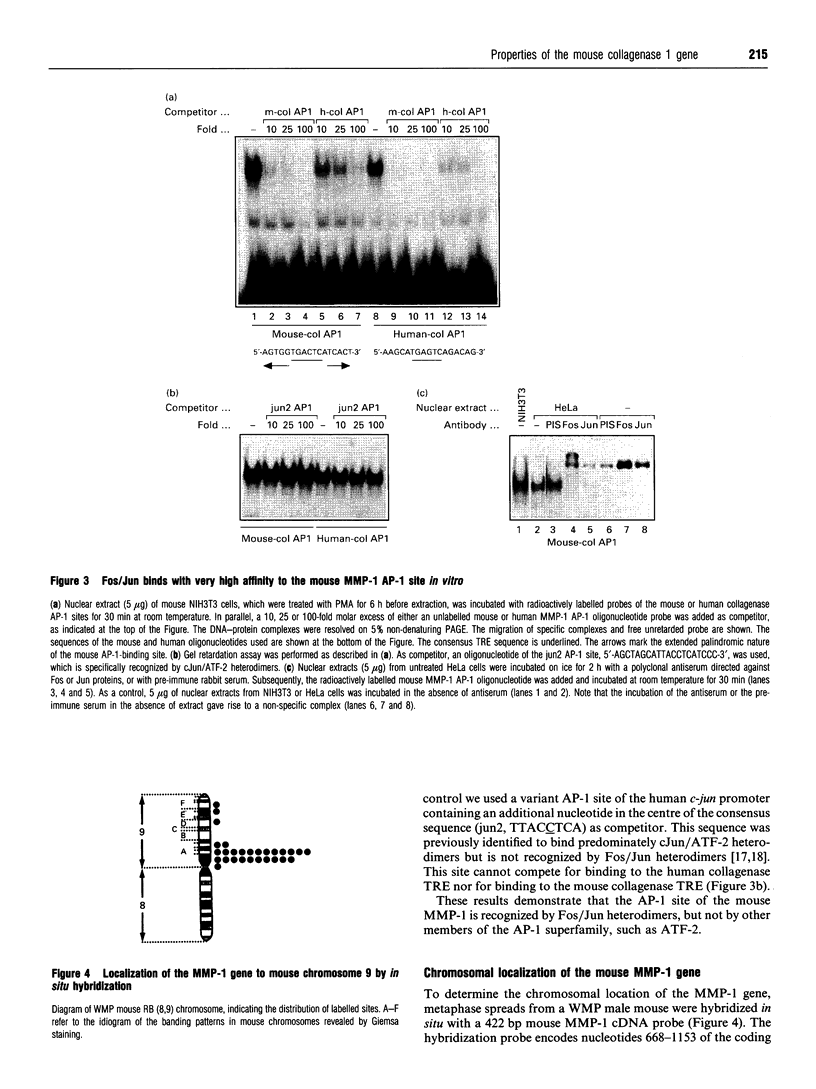
A clone containing genomic sequences of part of the murine collagenase type 1 (MMP-1) gene was isolated. It contains exons 1-6 encoding all the domains required for collagenase function and 9 kb of 5'-flanking sequences. The gene organization and exon/intron borders are highly similar to the already described human and rabbit MMP-1 genes. However, neither the intron sequences, nor the promoter region up to position -660 exhibit significant sequence homologies with rabbit and human MMP-1, except for an AP-1-binding site and two PEA-3 consensus sequences. Binding studies in vitro revealed that the AP-1-binding site is recognized by Fos/Jun heterodimers with very high affinity. By in situ hybridization the mouse MMP-1 gene was located to the A1-A2 region of chromosome 9 in proximity to the curly whiskers (cw) locus. Based on the lack of sequence homologies of the promoter and intron regions, and since the chromosomal localization of the mouse and human MMP-1 genes may not be syntenic, these data strongly support previous suggestions that the MMP-1 genes from mouse, compared with rabbit and human, have evolved from different ancestral genes. The presence of the AP-1- and PEA-3- binding sites in all mammalian MMP-1 genes isolated so far, may, however, suggest evolutionary selection for common regulatory mechanisms of MMP-1 transcription.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander C. M., Werb Z. Proteinases and extracellular matrix remodeling. Curr Opin Cell Biol. 1989 Oct;1(5):974–982. doi: 10.1016/0955-0674(89)90068-9. [DOI] [PubMed] [Google Scholar]
- Angel P., Baumann I., Stein B., Delius H., Rahmsdorf H. J., Herrlich P. 12-O-tetradecanoyl-phorbol-13-acetate induction of the human collagenase gene is mediated by an inducible enhancer element located in the 5'-flanking region. Mol Cell Biol. 1987 Jun;7(6):2256–2266. doi: 10.1128/mcb.7.6.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Angel P., Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991 Dec 10;1072(2-3):129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- Birkedal-Hansen H., Moore W. G., Bodden M. K., Windsor L. J., Birkedal-Hansen B., DeCarlo A., Engler J. A. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4(2):197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fini M. E., Plucinska I. M., Mayer A. S., Gross R. H., Brinckerhoff C. E. A gene for rabbit synovial cell collagenase: member of a family of metalloproteinases that degrade the connective tissue matrix. Biochemistry. 1987 Sep 22;26(19):6156–6165. doi: 10.1021/bi00393a032. [DOI] [PubMed] [Google Scholar]
- Formstone C. J., Byrd P. J., Ambrose H. J., Riley J. H., Hernandez D., McConville C. M., Taylor A. M. The order and orientation of a cluster of metalloproteinase genes, stromelysin 2, collagenase, and stromelysin, together with D11S385, on chromosome 11q22-q23. Genomics. 1993 Apr;16(1):289–291. doi: 10.1006/geno.1993.1181. [DOI] [PubMed] [Google Scholar]
- Gack S., Vallon R., Schaper J., Rüther U., Angel P. Phenotypic alterations in fos-transgenic mice correlate with changes in Fos/Jun-dependent collagenase type I expression. Regulation of mouse metalloproteinases by carcinogens, tumor promoters, cAMP, and Fos oncoprotein. J Biol Chem. 1994 Apr 8;269(14):10363–10369. [PubMed] [Google Scholar]
- Gutman A., Wasylyk B. The collagenase gene promoter contains a TPA and oncogene-responsive unit encompassing the PEA3 and AP-1 binding sites. EMBO J. 1990 Jul;9(7):2241–2246. doi: 10.1002/j.1460-2075.1990.tb07394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriet P., Rousseau G. G., Eeckhout Y. Cloning and sequencing of mouse collagenase cDNA. Divergence of mouse and rat collagenases from the other mammalian collagenases. FEBS Lett. 1992 Sep 28;310(2):175–178. doi: 10.1016/0014-5793(92)81323-e. [DOI] [PubMed] [Google Scholar]
- Herr I., van Dam H., Angel P. Binding of promoter-associated AP-1 is not altered during induction and subsequent repression of the c-jun promoter by TPA and UV irradiation. Carcinogenesis. 1994 Jun;15(6):1105–1113. doi: 10.1093/carcin/15.6.1105. [DOI] [PubMed] [Google Scholar]
- Kerr L. D., Holt J. T., Matrisian L. M. Growth factors regulate transin gene expression by c-fos-dependent and c-fos-independent pathways. Science. 1988 Dec 9;242(4884):1424–1427. doi: 10.1126/science.2462278. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Steeg P. S., Stetler-Stevenson W. G. Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell. 1991 Jan 25;64(2):327–336. doi: 10.1016/0092-8674(91)90642-c. [DOI] [PubMed] [Google Scholar]
- Matrisian L. M. Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet. 1990 Apr;6(4):121–125. doi: 10.1016/0168-9525(90)90126-q. [DOI] [PubMed] [Google Scholar]
- Mattei M. G., Philip N., Passage E., Moisan J. P., Mandel J. L., Mattei J. F. DNA probe localization at 18p113 band by in situ hybridization and identification of a small supernumerary chromosome. Hum Genet. 1985;69(3):268–271. doi: 10.1007/BF00293038. [DOI] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponta H., Cato A. C., Herrlich P. Interference of pathway specific transcription factors. Biochim Biophys Acta. 1992 Feb 11;1129(3):255–261. doi: 10.1016/0167-4781(92)90501-p. [DOI] [PubMed] [Google Scholar]
- Schorpp M., Kugler W., Wagner U., Ryffel G. U. Hepatocyte-specific promoter element HP1 of the Xenopus albumin gene interacts with transcriptional factors of mammalian hepatocytes. J Mol Biol. 1988 Jul 20;202(2):307–320. doi: 10.1016/0022-2836(88)90460-3. [DOI] [PubMed] [Google Scholar]
- Spurr N. K., Gough A. C., Gosden J., Rout D., Porteous D. J., van Heyningen V., Docherty A. J. Restriction fragment length polymorphism analysis and assignment of the metalloproteinases stromelysin and collagenase to the long arm of chromosome 11. Genomics. 1988 Feb;2(2):119–127. doi: 10.1016/0888-7543(88)90093-6. [DOI] [PubMed] [Google Scholar]
- van Dam H., Duyndam M., Rottier R., Bosch A., de Vries-Smits L., Herrlich P., Zantema A., Angel P., van der Eb A. J. Heterodimer formation of cJun and ATF-2 is responsible for induction of c-jun by the 243 amino acid adenovirus E1A protein. EMBO J. 1993 Feb;12(2):479–487. doi: 10.1002/j.1460-2075.1993.tb05680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]