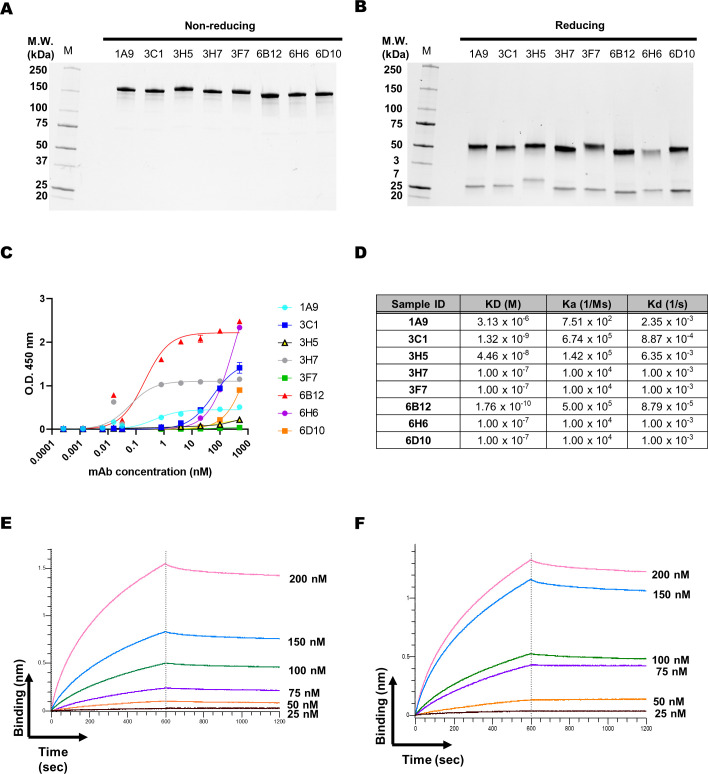
Figure 3.
Antibody production and characterization. Two IgG antibodies were purified from hybridoma and six IgG antibodies from an immune library. The expressed antibodies were purified using a protein A column and analyzed by SDS-PAGE to determine their sizes and purity. (A) The non-reducing condition showed an intact size of approximately 150 kDa, while (B) the reducing condition showed sizes of 50 kDa (Heavy chain) and 25 kDa (Light chain). (C) The binding activity of the eight IgG antibodies was validated using recombinant FopA protein. ELISA assessed the dose-dependent binding of 6B12, 3H7, and 3C1 IgGs. (D) The binding affinity of the eight antibodies was measured using biolayer interferometry. Recombinant FopA was immobilized on an AR2G biosensor and allowed to bind to the antibodies. (E, F) For detailed analysis, the antibodies were immobilized on an AR2G biosensor and subsequently permitted to bind with the FopA antigen diluted to various concentrations (25, 50, 75, 100, 150, and 200 nM). Kinetic rates and equilibrium binding constants were analyzed using global fitting analysis of the binding curves. Values represent the mean ± SD for a duplicate (C).