Abstract
In traditional Chinese medicine (TCM), based on various pathogenic symptoms and the ‘golden chamber’ medical text, Huangdi Neijing, diabetes mellitus falls under the category ‘collateral disease’. TCM, with its wealth of experience, has been treating diabetes for over two millennia. Different antidiabetic Chinese herbal medicines reduce blood sugar, with their effective ingredients exerting unique advantages. As well as a glucose lowering effect, TCM also regulates bodily functions to prevent diabetes associated complications, with reduced side effects compared to western synthetic drugs. Chinese herbal medicine is usually composed of polysaccharides, saponins, alkaloids, flavonoids, and terpenoids. These active ingredients reduce blood sugar via various mechanism of actions that include boosting endogenous insulin secretion, enhancing insulin sensitivity and adjusting key enzyme activity and scavenging free radicals. These actions regulate glycolipid metabolism in the body, eventually achieving the goal of normalizing blood glucose. Using different animal models, a number of molecular markers are available for the detection of diabetes induction and the molecular pathology of the disease is becoming clearer. Nonetheless, there is a dearth of scientific data about the pharmacology, dose‐effect relationship, and structure–activity relationship of TCM and its constituents. Further research into the efficacy, toxicity and mode of action of TCM, using different metabolic and molecular markers, is key to developing novel TCM antidiabetic formulations.
Keywords: animal model, Chinese herbal medicine, diabetes mellitus, evaluation index, mechanism of action
The endocrine disorder diabetes mellitus (DM) is a global phenomenon significantly influencing human health. Traditional Chinese medicine (TCM), with its long history, has been treating diabetes for over two millennia. The main types of animal used to model diabetes are rodents, zebrafish and primates. Using different animal models, the number of molecular markers available for the detection of diabetes induction, such as fasting blood sugar, postprandial blood glucose, glycated hemoglobin, glucose tolerance test, and lipid metabolism abnormalities, among others, is increasing. In addition, different antidiabetic Chinese herbal medicines reduce blood sugar and their effective ingredients have unique advantages. Besides their glucose lowering effects, antidiabetic TCM formulations also regulate human bodily functions to prevent diabetes associated complications, with reduced side effects compared to western synthetic drugs. The active ingredients of Chinese herbal medicine are mainly polysaccharides, saponins, alkaloids, flavonoids, terpenoids. These ingredients act via various mechanisms, including boosting endogenous insulin secretion, enhancing insulin sensitivity, adjusting key enzyme activity and scavenging free radicals, which regulate glycolipid metabolism in the body, to eventually normalize blood glucose.
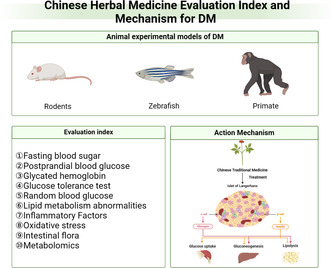
1. INTRODUCTION
The endocrine disorder diabetes mellitus (DM) is a global phenomenon, with a significant effect on human health. 1 The International Diabetes Federation (IDF) reported that 537 million people between the ages of 20 and 79 years were affected in 2021 and they predicted that this number will reach 643 million by 2030. 2 The typical clinical symptoms of DM include, in TCM terms, ‘three enhances with one reduction’, i.e., significantly enhanced thirst, urination, and appetite with significant weight loss. Diabetes is characterized by hyperglycemia, hyperinsulinemia, insulin resistance with elevated oxidative stress and inflammation (Figure 1). It impairs carbohydrate, fat and protein metabolism thus causing various degrees of micro‐ and macro‐vascular deterioration in core organs of the body. Reports have suggested that diabetes is positively correlated with neuropathological disorders, cardiomyopathy, nephropathy, gastrointestinal or genitourinary tract complications and non‐healing wounds. Diabetes with progressive end organ complications further worsens the quality of life and is ranked 9th among the top causes of mortality in the world. It is also a huge economic burden. The IDF reported that in 2021 966 billion USD was spent to treat diabetes and related complications.
FIGURE 1.
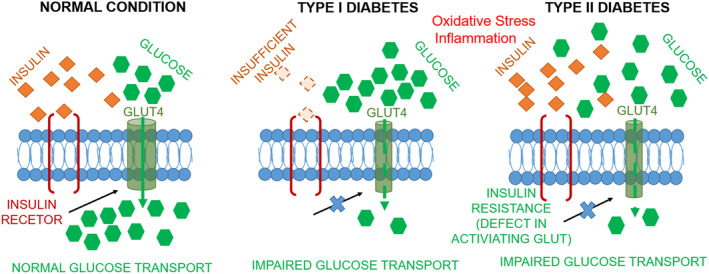
Causes of type I and type II diabetes.
Antidiabetic therapies in western medicine include metformin, sulfonylureas, glitazone, and DPP‐IV inhibitors, among others. Beside their beneficial effects they also have several side effects including hypoglycemia, gastrointestinal distress, liver and kidney dysfunction. 3 Considering these disadvantages, researchers are seeking novel antidiabetic formulations with better efficiency and less toxicity. Natural products have proved to be a good source of novel antidiabetic compounds. Traditional Chinese Medicine, with its long history, is one of the possibilities for providing new antidiabetic formulations with long‐lasting effects and fewer adverse reactions that can delay diabetes or its related complications.
Different animal models provide a way of investigating specific aspects of the pathophysiology of diabetes such as hyperglycemia and insulin insufficiency or resistance, and their association with end organ complications, as well as elucidating the antidiabetic effects of antidiabetic drugs. Use of such models has led to the exploration of the therapeutic potential of TCM agents in treating diabetes, and laid the foundation for the efficient utilization, patenting, and manufacture of TCM antidiabetic formulations. In this review, we have investigated the different diabetes model available and highlighted the therapeutic potential of TCM compared to western medicine.
2. ANIMAL EXPERIMENTAL MODELS OF DM
2.1. Type I diabetes model in rodents
This model is also known as pancreatic islet injury hyperglycemia model in rodents. It is induced chemically, spontaneously and by genetic modification, resulting in the destruction of the insulin‐secreting pancreatic β cells, which leaves the rodent in a chronic state of hyperglycemia through lack of enough insulin to reduce blood glucose. Type I diabetes is manifested by frequent urination, increased thirst, hunger, and weight loss. Different type I diabetes models induced chemically, via genetic manipulation and phenotypically are listed in Table 1.
TABLE 1.
Diabetic model of islet damage in rodents.
| Induction mechanism | Model | Induction mechanism | Model |
|---|---|---|---|
| Chemical induction | Streptozocin (single injection 150–200 mg/kg or injections 50 mg/kg for 5 days) | Genetic induction | Akita mouse |
| Alloxan (single injection 100–200 mg/kg) | Spontaneous autoimmunity | NOD mouse | |
| Virus induction | Coxsackie B virus | BB mouse | |
| Cerebral myocarditis virus | LEW.1NR1/ztm‐iddm rat | ||
| Kilham rat virus | Lewis‐IDDM rat | ||
| LCMV insulin promoter | KDP rat |
2.2. Type II diabetes model in rodents
This model is also referred as the insulin resistance or glucose/lipid metabolism disorder model. It creates an endocrine disorder, usually induced by a high fat and high sugar diet with or without low doses of streptozotocin (STZ). Type II diabetes is characterized by β‐cell dysfunction and insulin resistance (IR). Its primary symptoms include increased appetite and thirst, weariness, impaired vision, frequent urination, and weight loss. The different methods used to induce type II diabetes in rats via diet, chemical intervention, or genetic modification are listed in Table 2. 4
TABLE 2.
Insulin resistance glucose\lipid metabolism disorder model in rodents.
| Induction mechanism | Model | Induction mechanism | Model | |
|---|---|---|---|---|
| Induction type | Drug induction | Streptozocin(15–30 mg/kg) | Transgenic type | GK/IRS‐1 double knockout |
| Intravenous BCG vaccine | INS knockout | |||
| Dexamethasone | Lipoproteinase Knockout | |||
| Monosodiumglutamate | SH2‐B knockout | |||
| Milrinone | CAV3 knockout | |||
| Intravenous lipid compounds | IGF‐1 knockout | |||
| Food induction | High Fat Diet Feeding | Gck / Hnf4a / Abcc8 knockout | ||
| High sugar feeding | PD‐1 gene overexpression | |||
| High‐fat high‐sugar mixed feeding | Genetic type | KK‐Ay, db/db, ob/ob, NSY mice | ||
| Diet plus medication induction | High‐fat diet + Streptozocin | ZDF, OLETF rats | ||
| High‐fat diet + Alloxan | GK, NOZ rats | |||
| Surgical induction | Pancreatectomy | Zuker obese rats | ||
| Streptozocin + Pancreatectomy | Wistar obese rats | |||
2.3. Other animal models
Zebrafish can serve as an ideal model to study diabetes pathophysiology or the antidiabetic effects of novel formulations owing to their small body size, large spawning capacity, short growth cycle, and simple mode of adult reproduction. 5 Zebrafish share 87% genetic homology with humans. 5 They also feature some key mechanisms for the regulation of glucose metabolism that are similar to other mammals, thus making them a good candidate model for studying diabetes. 6 The type I diabetes model in zebrafish can be induced by surgical removal of the pancreas, chemically inducing β‐cell apoptosis, or using transgenic techniques. 7 In contrast, type II diabetes is induced via environmental factors, gene modification or a high fat, high glucose diet. 8 , 9
Non‐human primates due to their closer genetic similarity can better mimic the pathophysiology of human diabetes mellitus. Animal models of spontaneous diabetes in non‐human primates include squirrel monkeys (Saimiri sciureus), crab‐eating monkeys (Macaca fascicularis), 10 rhesus monkeys (M. mulatta), 11 Sulawesi monkeys (M. nigra), tree shrews (Tupaia belangeri), 12 baboons (Papio hamadryas), chimpanzees (Pan troglodytes), Taiwan macaques (M. cyclopis), and gray baboons (Mandrillus leucophaeus). 13 Type I diabetes in non‐human primate is induced by STZ 14 with or without pancreatectomy, 15 while type II diabetes is induced by a high energy diet with STZ. 16 Transgenic macaques can be obtained commercially and can be especially useful as their genome has been sequenced. 17
3. EVALUATION INDEX
3.1. Human clinical evaluation indexes
The main indicators of human clinical diabetes include fasting glucose and 2‐hour postprandial glucose, 18 insulin sensitivity, intestinal diabetic markers and related peptides, glycated protein, glycated hemoglobin (HbA1c), 19 hemorheological parameters (plasma viscosity, low shear whole blood viscosity, sedimentation rate and red blood cell deposition), 20 glycated serum protein, 1,5‐anhydroglucitol (1,5‐AG), total cholesterol, and triglycerides 21 (see Table 3).
TABLE 3.
Characteristics of diabetic evaluation indexes.
| Indicators | Description | Advantages | Disadvantages |
|---|---|---|---|
| Fasting blood sugar | After 8 hours of fasting, blood glucose levels were tested |
Easy to measure Allows rapid assessment of insulin secretion Important for diabetes screening |
Highly influenced by diet, fasting status required Not suitable for evaluation of hypoglycemia |
| Postprandial blood glucose | Tested 2 h after eating and drinking to determine blood glucose levels. |
Helps assess insulin resistance and insulin secretion capacity Important for postprandial glucose control in diabetic patients |
Requires standardized pre‐meal diet Not easy to compare results at different time points |
| Glycated hemoglobin | Reflects the average level of glucose in red blood cells and is usually measured once in 3–4 months |
Represents the average blood sugar levels during a three‐to‐four‐month period. Can predict the risk of diabetic complications |
Requires regular testing Interfered by factors such as anemia |
| Glucose tolerance test | Measured 2 h after ingesting 75 g of glucose orally on an empty stomach to determine blood glucose concentration |
Can detect underlying diabetes Helps assess insulin secretion function |
Requires large oral doses of glucose Long testing time |
| Hemorheological parameters | It mainly reflects the changes in blood fluidity, stagnation and blood viscosity caused by changes in blood composition | Can predict the risk of diabetic complications | Highly influenced by diet, fasting status required |
| Random blood glucose | Measure blood glucose concentration at any time |
Easy to measure Allows rapid assessment of blood glucose levels |
Highly influenced by diet Cannot assess insulin secretion |
| Lipid metabolism abnormalities | Abnormalities in lipid metabolism are usually associated with diabetes and include indicators such as HDL‐C, LDL‐C, and TC. |
Assess cardiovascular risk in diabetic patients Can help to understand insulin resistance |
Affected by factors such as diet and exercise Not suitable for all diabetic patients |
| Inflammatory Factors | Testing for inflammation‐related indicators can help to understand the health status of diabetic patients |
Can assess the degree of inflammation in diabetic patients Can help understand the risk of diabetes complications |
Affected by factors such as diet and exercise Not suitable for all diabetic patients |
| Oxidative stress | Can result in high levels of oxidative stress because of things like hyperglycemia, which raises the risk of complications from diabetes. |
Can assess the degree of oxidative stress in diabetic patients Can help understand the risk of diabetic complications |
The testing procedure is complicated and necessitates the use of specialist equipment and abilities. Not suitable for all diabetic patients |
| Intestinal flora | The intestinal flora may be altered due to factors such as diet structure, which increases the risk of diabetic complications. |
Can help to understand the intestinal microecology of diabetic patients Can predict the risk of diabetic complications |
Complex test method, requires specialized equipment and skills Additional research support is needed |
| Metabolomics | Can reflect the various chemical reactions that occur when the body status of diabetic patients changes, providing a novel means of diagnosis and treatment of diabetes. |
Highly sensitive Non‐invasive Comprehensive |
Sample processing issues Complex data processing Expensive equipment costs |
3.2. Animal model evaluation indexes
The general indicators used to evaluate the antidiabetic effect of novel formulations in islet injury or insulin resistance models include fasting blood glucose, postprandial hyperglycemia, serum biochemical indicators, insulin level, and others. 22 Physical parameters include polydipsia, polyphagia, polyuria, and weight loss. Diabetes is a metabolic disorder so, the evaluation of serum biochemical markers such as total cholesterol (TC), triglyceride (TG), low density lipoprotein cholesterol (LDL‐C) and high‐density lipoprotein cholesterol (HDL‐C), blood creatinine (CR), blood urea nitrogen (BUN), alanine aminotransferase (Alt), aspartate aminotransferase (AST) is of fundamental importance. Pathological biopsies from pancreatic and other organs involved are performed to observe tissue destruction. Fasting serum insulin levels, serum insulin levels, insulin sensitivity index, glucose tolerance are also other important markers to evaluate diabetes progression. Diabetes is characterized by significant elevated oxidative stress and inflammation, thus the level of related indicators such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH‐Px), reactive oxygen species (ROS), interleukin‐6 (IL‐6), interleukin‐1β (IL‐1β), and tumor necrosis factor‐α (TNF‐α), interferon‐γ (IFN‐γ) 23 is worth monitoring. The modulation of gut microflora can also be an effective evaluation marker for an antidiabetic therapy (Table 3). 24
3.3. In vitro experiments evaluation indexes
There are two major methods for evaluating in vitro antidiabetic models, enzyme and cellular assays, so the evaluation index varies with the assay used. The enzymatic approach usually evaluates the inhibition of α‐amylase and α‐glucosidase in the presence of antidiabetic compounds, 25 and can be done using a molecular docking approach or an enzyme inhibition assay. This method has the limitation that it fails to predict activity in the whole‐animal environment, and so lacks any clinical and safety evaluations, but it is a robust preliminary method for screening a library of compounds and formulations for antidiabetic activity.
Researchers usually replicate enzyme inhibition data in cellular models before testing them in in vivo models. In cellular models, the biological effects of the active material are tested, and they are widely used as active assessment systems. The main evaluation indicators include oxidative stress, lipid metabolism, followed by gene or protein expression of key enzymes in glucose and lipid metabolism (superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH‐Px), reactive oxygen species (ROS), 26 DPPH radical scavenging capacity, ABTS+ radical scavenging capacity, and hydroxyl radical scavenging capacity 27 ), glucose metabolism (glucose kinase (GK), 28 glucose‐6‐phosphatase (G6Pase), 29 pyruvate kinase (PK), hexokinase (HK), 25 protein tyrosine phosphatase 1B (PTP‐1B), 30 etc.), relevant signaling pathways (PI3K/Akt, 31 MAPK, 32 cAMP‐PKA, 33 AMPK signaling pathways, 34 ) etc. (Table 3).
These indicators can be used to evaluate the antidiabetic efficacy of TCM in various in vitro, pre‐clinical and clinical studies.
4. TRADITIONAL CHINESE MEDICINES AS ANTIDIABETIC AGENTS
4.1. Glucose lowering effect of herbal and Chinese medicinal compounds
TCM herbal formulations and single compounds significantly alleviate the pathophysiology and evaluation indexes of diabetes. These initial findings form the basis of future continuous improvement in TCM formulations and exploration of new glucose lowering TCMs by focusing on different pathogenic mechanisms of diabetes. The main categories of herbs for lowering blood sugar are tonic, heat‐clearing and blood‐activating, mainly including Astragali Radix, 35 Cinnamomi Cortex, 36 Ginseng Radix, 37 Lycii Fructus, 38 Salviae Miltiorrhizae Radix et Rhizoma, 39 Rehmanniae Radix, 40 Corn Stigma, 41 Anemarrhenae Rhizoma, 42 Angelicae Sinensis Radix, 43 Puerariae Lobatae Radix, 44 Coptidis Rhizoma, 45 Dioscoreae Rhizoma, 46 Polygonati Odorati Rhizoma, 47 Poria, 48 Cuscuta europaea, 49 Atractylodis Rhizoma, 50 Atractylodis Macrocephalae Rhizoma, 51 Agrimoniae Herba, 52 Xanthii Fructus, 53 Alismatis Rhizoma, 54 Platycodonis Radix, 55 Polygonati Rhizoma, 56 Morus alba L., 57 Momordica charantia L., 58 etc.
Usually, TCM formulations are multidirectional and multitargeted, which makes them more effective than chemically synthesized drugs. At present, the Pharmacopeia of the People's Republic of China and the Ministerial Standards includes more than a dozen glucose lowering Chinese patent medicines, including Xiaoke Ling tablets, Thirsty Lening tablets, Jinqi Jiangtang tablets, Yuye Xiaoke granules, Yangyin Jiangtang tablets, Jiangtang A tablets, Jiangtang Shu capsules, Xiaoke Jiangtang tablets, Jiangtang capsules, Xiaoke Jiangtang capsules, Xiaoke pills, etc. 59 Zhenqi antidiabetic capsules are internationally recognized and are mainly refined from Ginseng Radix, Astragalus membranaceus, Polygonati Rhizoma, pearl, and other rare Chinese medicinal herbs. A meta‐analysis of treatments for diabetic nephrotoxicity comparing conventional western medicine treatment groups with patients receiving western medicine in conjugation with Bushen Huoxue decoction showed that Bushen Huoxue decoction has certain advantages in lowering blood sugar, regulating blood lipid and reversing renal function when given in combination with western treatments compared to western medicine alone. 60
At present, many simulation experiments with different blood glucose indicators, conducted in a variety of models, have provided many diabetes assessment indicators, which form the basic evaluation indexes for research on the hypoglycemic effects of TCM. Diabetes often causes complications in multiple tissues and organs that seriously endanger life and health and require long‐term medication and dietary attention. The pathogenesis of diabetes mellitus is very complex, and the pathogenesis of different types of diabetes mellitus is also clinically different. Diabetes is often caused by a variety of factors, and its pathogenesis is composed of multiple syndromes. Therefore, continuing studies aimed at discovering the pathogenesis of diabetes mellitus and related evaluation indicators are needed to improve the evaluation index system for glucose reduction by TCM.
4.2. Traditional Chinese medicine active ingredients and their antidiabetic efficacy
There are many active ingredients of TCM with proven glucose lowering effects, including polysaccharides, saponins, alkaloids, flavonoids and terpenoids. The glucose lowering effects of these constituents are multifactorial but are broadly of four types: (1) to inhibit of pancreatic β cell apoptosis or repair β cells to restore insulin production; (2) to enhance insulin sensitivity toward target cells; (3) to maintain the key enzyme activity (α amylase, α glycosidase enzymes) to enhance sugar metabolism and avoid reabsorption; (4) to scavenge free radicals, enhance antioxidant capacity and prevent lipid peroxidation. (Table 4).
TABLE 4.
Diabetic active ingredients and mechanism of action of Chinese medicines.
| Active ingredients | Chinese medicine | Action mechanism | Mechanism | Reference |
|---|---|---|---|---|
| Polysaccharides | Ginseng | Fasting blood glucose ↓, MDA ↓, insulin ↑, SOD activity ↑, liver glycogen content ↑ | (1, 4) | [61] |
| G6P activity ↓, hepatic glycogen synthase activity ↓, G6PD activity ↑, PFK activity ↑ | (3) | [62] | ||
| Lycii Fructus | HbA1c, GSP, insulin level, TC, TG, LDL‐C ↓; HDL‐C ↑; GLP‐1, PYY, GPR41, GPR43 expression ↑, GSK‐3β and PEPCK expression ↓, activation of InsR/PI3K/AKT signaling pathway | (2, 4) | [63] | |
| Vigna umbellata | Body weight ↓, fasting blood glucose ↓, TG↓, HDL‐C↑; Expression of INSR, IRS‐1, PI3K, AKT, and GLUT‐2 ↑ activates the PI3K/AKT signaling pathway | (2, 4) | [64] | |
| Astragali Radix | Body weight, fasting glucose, TC, TG, LDL‐C ↓, HDL‐C ↑, antioxidant enzyme activity ↑, MDA ↓ | (2) | [35] | |
| Ophiopogonis Radix | Expression of PI3K, AKT, InsR, PPARγ ↑; expression of PTP‐1B at mRNA level and protein level ↓ | (2, 3) | [65] | |
| Lowering blood sugar levels, increasing insulin levels, repairing islet destruction and damage to pancreatic beta cells | (1) | [66] | ||
| Morus alba L. | Inhibited apoptosis, improved insulin secretion capacity of pancreatic β‐cells, and increased mRNA and protein expression of PDX‐1 and its downstream targets GLUT2 and GCK in pancreatic islet cells of diabetic rats. | (1, 3) | [67] | |
| Saponins | Fructus Momordicae | FBG ↓, serum insulin ↑, mRNA levels of G6Pase and PEPCK ↑, fat oxidation‐related genes ↑, activation of AMPK signaling pathway | (2, 3) | [68] |
| Ginseng | IRS‐1/PI3K/AKT insulin signaling pathway ↑, glucose uptake ↑; MAPK signaling pathway ↓, insulin resistance ↓ | (2) | [69] | |
| Sanchi | Blood glucose and serum insulin levels ↓, mRNA expression of p‐PI3K, p‐AKT, IRS1 and GLUT4 ↑, IRS1‐PI3K‐AKT signaling pathway ↑ | (1) | [70] | |
| Melandryum viscidulum | mRNA expression of Glut4, IRS‐1, Akt and PI3K ↑, mRNA expression of Moat1, Lipc, Lpcat4 and Smpd4 ↓, PI3K‐AKT signaling pathway ↑ | (1) | [71] | |
| Corni Fructus | IL‐2, TNF‐α and CRP ↑, MDA ↓, SOD activity ↑, mRNA expression of p‐PI3K, p‐AKT, IRS1 and GLUT4 ↑, IRS1‐PI3K‐AKT signaling pathway ↑ | (2, 4) | [72] | |
| Lily | Stimulation of glucose depletion in the liver with ↑ PPARγ expression induces GLUT4 translocation to the cell surface | (2) | [73] | |
| Alkaloids | Morus alba L. | Activation of insulin receptor pathway and TGF‐β/Smads signaling pathway to improve insulin resistance and oxidative stress‐induced hyperglycemia | (2) | [74] |
| Coptidis Rhizoma | GLP‐1 ↑; glucagonogen mRNA expression ↑ | (2, 3) | [75] | |
| Tinosporae Radix | Blocking K‐ATP channels to stimulate insulin secretion | (1) | [76] | |
| Flavonoids | Polygonati Odorati Rhizoma | Fasting blood glucose ↓, insulin ↑, α‐amylase activity ↓ | (1, 3) | [77] |
| India Madder Root | Scavenging of free radicals, SOD and catalase expression ↓, phosphorylation of IKK ↓ | (4) | [78] | |
| Chinese Angelica | α‐amylase and ACAT activities ↓, TC and TG levels ↓ | (3, 4) | [79] | |
| Liquorice root | FBG ↓, SOD ↑, MDA ↓ | (4) | [80] | |
| Ginkgo biloba Linn | α‐amylase and α‐glucosidase activities↓ | (3) | [81] | |
| Tangerine Peel | GK mRNA level ↑, PCK and G6P mRNA expression ↓, GLUT2 protein expression ↓, GLUT4 and PGC‐1 expression ↑ | (3) | [82] | |
| Terpenoids | Cyclocarya paliurus | Regulation of insulin resistance pathway, PI3K‐Akt signaling pathway, HIF‐1 signaling pathway, PPAR signaling pathway | (2, 4) | [83] |
| Forsythiae Fructus | Scavenging free radicals, protecting and repairing pancreatic beta cells | (1) | [84] | |
| Polygoni Multiflori Radix | Improvement of insulin resistance, insulin sensitivity↑ | (2) | [85] | |
| Dan‐Shen Root | α‐Glucosidase and α‐amylase activities↓ | (3) | [86] | |
| Croton Tiglium | Glucose uptake ↑, alleviating insulin resistance | (2) | [87] | |
| Gynostemma pentaphyllum | 2‐NBDG uptake ↑, GLUT4 translocation, AMPK and ACC signaling pathways ↑ | (2, 3) | [88] |
Note: ↑cytokine or pathway upregulation; ↓cytokine or pathway downregulation.
In recent years, great progress has been made in understanding the mechanism of action of TCMs as glucose lowering agents. Many Chinese medicinal herbs have been found to be active in controling diabetes and its associated complications. 89 , 90 The molecular mechanisms behind the antidiabetic effect of active TCM components have been studied. Comparative analyses show that the antidiabetic mechanisms of different TCMs are different; even the same active ingredient exerts its effect differently in different formulations. 61 , 62 So there is a compelling need to evaluate scientifically the mechanism of action of the active ingredients in different formulations to identify the most appropriate one in the formulation.
5. SAFETY OF TRADITIONAL CHINESE MEDICINE IN DIABETES TREATMENT AND COMPARISON WITH WESTERN MEDICINE
In western medicine, diabetes is mainly treated by diabetes education, medical nutrition therapy, exercise therapy, drug therapy and blood glucose monitoring. Drugs for the treatment of diabetes mainly include oral drugs and injectable preparations. Among them, the classic drugs include metformin, sulfonylureas, and thiazolidinediones, and the new drugs include GLP‐1 RA, DPP‐4i, and SGLT‐2i, which have different and rapid mechanisms of action, but also have different side effects 91 (Table 5).
TABLE 5.
Classification and side effects of western medicine treatment of diabetes.
| Drug type | Ensample | Target organ | Mode of action | Side effect |
|---|---|---|---|---|
| Insulin and insulin analogue | Insulin, Lispro, Aspart, Glulisine, Glargine, Aspart30 |
Liver Adipose Tissue Skeletal muscle |
Mimics normal insulin secretion |
Hypoglycemic response Allergic reaction |
| Biguanide | Metformin |
Muscle Liver Intestine |
Enhance glucose uptake and absorption Enhanced GLP‐1 Decrease gluconeogenesis |
Abdominal Discomfort Metallic taste Nausea Diarrhea Lowered eGFR Vitamin B12 Deficiency |
| Sulfonylureas | DiaBeta、Amaryl | Pancreas | Enhance Insulin Secretion |
Weight Gain Dizziness |
| Thiazolidinedione (TDZs) | Avandia, Actos |
Pancreas Muscle Adipose Tissue |
Enhance glucose uptake Enhance Insulin Sensitivity |
Weight gain Edema The risk of fracture is increased Increased risk of heart problems Bladder cancer |
| Glinide | Repaglinide, Nateglinide | β cell | Triggers the release of insulin from the pancreas |
Blood sugar levels drop too low Weight gain Rash Nausea Vomiting |
| Dipeptidyl peptidase 4 inhibitor (DPP‐4i) | Onglyza, Januvia, Tradjenta, Nesina |
Pancreas Intestine |
Reduced glucagon secretion Enhancing insulin secretion Enhance GLP‐1 |
Dizziness Acute pancreatitis Hepatic dysfunction Severe arthralgia Urinary Tract Infection Skin reaction |
| α‐Glucosidase inhibitors | Glyset, Acarbose | Intestinal tract | Slows the body's ability to break down starches and some sugars |
Flatulence Stomach pain Diarrhea |
| Sodium‐dependent glucose transporters 2 inhibitor (SGLT‐2i) | Invokana, Farxiga, Jardiance, Steglatro | Kidney | Enhanced glucose secretion |
Vulvogavinal candidiasis Urinary tract infections Hypotension Fatal urosepsis Diabetic ketoacidosis Pyelonephritis |
| Bile acid sequestrants | Chlestyramine, Colestipo | Gallbladder | Lower cholesterol |
Flatulence Constipation Dyspepsia The blood fat level increased |
| Glucagon‐like peptide‐1 receptor agonist (GLP‐1 RA) | Trulicity, Byetta, Adlyxin, Victoza |
Pancreas Intestine |
Reduced glucagon secretion Enhancing insulin secretion Gastric emptying |
Pancreatitis Nausea Vomiting Gallbladder disease Renal injury |
| 5‐ Aminolevulinic Acid (5‐ALA) | Pancreas | Enhance insulin scretion |
Diarrhea Menstrual Pain Headache |
Compared with western medicine, TCM mostly regulates the disease based on its cause thus helping the body to gradually improve, reducing the disease burden and reducing toxicity. Studies have shown that the overall effectiveness of Huangqi Xiaoji Huazhou decoction treatment in delaying the progression of diabetes is higher than in control patients, with fewer adverse reactions, thus making it an effective, safe and reliable candidate for antidiabetic therapy. 92 Similarly, in comparison to metformin, Jinlida granules can effectively improve blood glucose indicators with much lower adverse effects. 93 Moreover, TCM in combination with western medicine enhances the therapeutic efficacy of western medicine by reducing adverse effects. A literature survey showed that Danshen dripping pills in combination with western medicine significantly ameliorated diabetes related retinopathy compared to groups using western medicine alone. The treatment significantly normalized blood sugar and lipid profiles, with significantly reduced adverse effects among diabetic individuals. 94 The chance of contracting diabetes and its related end organ complications is higher in the elderly population. A strictly controlled dosage of Yiben Huoxue decoction, a traditional Chinese medicine, ensures normal glucose metabolism. 95 TCM alone or in combination can therefore be a serious intervention for the treatment of diabetes and its associated complications.
6. CONCLUSIONS AND PROSPECTS
The many varieties of TCM active ingredients with glucose lowering effects have unique advantages in the treatment of diabetes. They not only lower blood glucose, but also regulate human functions, prevent and treat complications, and avoid the toxic side‐effects of chemical synthetic drugs that lower blood glucose. With advancements in the development of biological models, the pathophysiology and molecular mechanisms of diabetes are becoming clearer over time. It is now important to take advantage of these advances to further elaborate the pharmacological relevance and molecular mechanisms of traditional and new antidiabetic TCM formulations. Scientific data from further clinical trials of TCM formulations in the treatment of diabetes will help to develop more targeted Chinese herbs and Chinese medicine compound for diabetes treatment. More research into the mechanisms of the various active ingredients of TCM formulations used to treat diabetes and its associated end organ complications is key to developing novel formulations. Preclinical studies using in vitro approaches to test the efficacy and toxicity of TCM formulations will aid the patenting of TCM formulations for treating diabetes and associated complications. Many natural products have both dietary and medicinal value. Chinese herbs provide another option for research into antidiabetic formulations, with a huge global potential market. In summary, the diabetic models described in this review can be used to evaluate TCM alone or in combination with western medicine to control the global problem of diabetes.
AUTHOR CONTRIBUTIONS
XL: Formal analysis, Writing‐Review & Editing; HZ: Formal analysis, Data Curation; TA: Formal analysis; FW: Data Curation, Supervision and funding support; BF:Data Curation, Supervision, Paper revision and funding support; QW: Data Curation, Supervision, Paper revision and funding support.
FUNDING INFORMATION
This work was supported by the National Key Research and Development Program of China (2022YFD1600303, 2021YFD1600100).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
ETHICS STATEMENT
Not applicable.
ACKNOWLEDGMENTS
We should thank the National Key Research and Development Program of China for their support. We would like to express their gratitude to. EditSprings (https://www.editsprings.cn) for the expert linguistic services provided.
Liu X‐Y, Zheng H‐W, Wang F‐Z, Atia T‐W, Fan B, Wang Q. Developments in the study of Chinese herbal medicine's assessment index and action mechanism for diabetes mellitus. Anim Models Exp Med. 2024;7:433‐443. doi: 10.1002/ame2.12455
Contributor Information
Bei Fan, Email: fanbei517@163.com.
Qiong Wang, Email: luyiwangqiong@163.com.
REFERENCES
- 1. Zhang L, Zhou X, Chen H, et al. Mulberry extract ameliorates T2DM‐related symptoms via AMPK pathway in STZ‐HFD‐induced C57BL/6J mice. J Ethnopharmacol. 2023;313:116475. doi: 10.1016/j.jep.2023.116475 [DOI] [PubMed] [Google Scholar]
- 2. Tönnies T, Rathmann W, Hoyer A, Brinks R, Kuss O. Quantifying the underestimation of projected global diabetes prevalence by the international diabetes federation (IDF) diabetes atlas. BMJ Open Diabetes Res Care. 2021;9(1):e002122. doi: 10.1136/bmjdrc-2021-002122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. ChienTung W, YuehTing T, HsuanKuang J, et al. Metformin and the risk of anemia of advanced chronic kidney disease in patients with type 2 diabetes mellitus. J Clin Pharmacol. 2021;62(2):276‐284. doi: 10.1002/jcph.1965 [DOI] [PubMed] [Google Scholar]
- 4. Xiangning H, Xinyi Y, Yan T, Rong Y. Application of animal models of type 2 diabetes mellitus: based on data mining. Chin J Exp Tradit Med Formulae. 2023;29(2):159‐165. doi: 10.13422/j.cnki.syfjx.20221511 [DOI] [Google Scholar]
- 5. Kongli H, Shijie S, Yuting W, et al. Research progress on model organism zebrafish in neurodegenerative diseases. Chin J Comp Med. 2023;33(10):121‐131. [Google Scholar]
- 6. Capiotti KM, Antonioli Junior R, Kist LW, Bogo MR, Bonan CD, Da Silva RS. Persistent impaired glucose metabolism in a zebrafish hyperglycemia model. Comp Biochem Physiol B Biochem Mol Biol. 2014;171:58‐65. doi: 10.1016/j.cbpb.2014.03.005 [DOI] [PubMed] [Google Scholar]
- 7. Kleinert M, Clemmensen C, Hofmann SM, et al. Animal models of obesity and diabetes mellitus. Nat Rev Endocrinol. 2018;14(3):140‐162. doi: 10.1038/nrendo.2017.161 [DOI] [PubMed] [Google Scholar]
- 8. Zang L, Maddison LA, Chen W. Zebrafish as a model for obesity and diabetes. Front Cell Dev Biol. 2018;6:91. doi: 10.3389/fcell.2018.00091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li Y, Chen Q, Liu Y, et al. High glucose‐induced ROS‐accumulation in embryo‐larval stages of zebrafish leads to mitochondria‐mediated apoptosis. Apoptosis. 2022;27(7–8):509‐520. doi: 10.1007/s10495-022-01731-2 [DOI] [PubMed] [Google Scholar]
- 10. Wu D, Zou C, Yue F, Li X, Li S, Zhang YA. The effect of long‐term streptozotocin‐induced diabetes mellitus (STZ‐DM) on cynomolgus (Macaca Fascicularis) monkeys. J Med Primatol. 2009;38(1):15‐22. doi: 10.1111/j.1600-0684.2008.00300.x [DOI] [PubMed] [Google Scholar]
- 11. Shibata S, Kirchhof N, Matsumoto S, et al. High‐dose streptozotocin for diabetes induction in adult rhesus monkeys. Transplant Proc. 2002;34(4):1341‐1344. doi: 10.1016/s0041-1345(02)02796-3 [DOI] [PubMed] [Google Scholar]
- 12. Wagner JD, Cline JM, Shadoan MK, Bullock BC, Rankin SE, Cefalu WT. Naturally occurring and experimental diabetes in cynomolgus monkeys: a comparison of carbohydrate and lipid metabolism and islet pathology. Toxicol Pathol. 2001;29(1):142‐148. doi: 10.1080/019262301301418955 [DOI] [PubMed] [Google Scholar]
- 13. Bin L, XiaoYu W. Progress on nonhuman primate models of diabetes mellitus. Zool Res. 2011;32(1):91‐96. doi: 10.3724/SP.J.1141.2011.01091 [DOI] [PubMed] [Google Scholar]
- 14. Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res. 2001;50(6):537‐546. [PubMed] [Google Scholar]
- 15. Qiao CF, Tian BL, Mai G, et al. Induction of diabetes in rhesus monkeys and establishment of insulin administration strategy. Transplant Proc. 2009;41(1):413‐417. doi: 10.1016/j.transproceed.2008.08.144 [DOI] [PubMed] [Google Scholar]
- 16. Wagner JD, Kavanagh K, Ward GM, Auerbach BJ, Harwood HJ Jr, Kaplan JR. Old world nonhuman primate models of type 2 diabetes mellitus. ILAR J. 2006;47(3):259‐271. doi: 10.1093/ilar.47.3.259 [DOI] [PubMed] [Google Scholar]
- 17. Niu Y, Yu Y, Bernat A, et al. Transgenic rhesus monkeys produced by gene transfer into early‐cleavage‐stage embryos using a simian immunodeficiency virus‐based vector. Proc Natl Acad Sci USA. 2010;107(41):17663‐17667. doi: 10.1073/pnas.1006563107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brennan M, Crowe A, Tiernan C, et al. Risk of Hypoglycaemia in older patients in residential care on Oral Hypoglycaemic medication. Age Ageing. 2019;48:iii17‐iii65. doi: 10.1093/ageing/afz103.107 [DOI] [Google Scholar]
- 19. Huan Y, Hong C, Mi Z, Dongfang W, Qiaoli J. Establishment of the quality index of clinical pharmacists in chronic disease Management for Patients with type 2 diabetes. China Pharmacist. 2019;22(11):2049‐2051. [Google Scholar]
- 20. Zhengzhuan J, Liping Z. Evaluation of blood lipid indicators, Hemorheology for the clinical effectiveness of diabetes tests. Diabetes New World. 2023;26(17):57‐60. doi: 10.16658/j.cnki.1672-4062.2023.17.057 [DOI] [Google Scholar]
- 21. Singh RG, Nguyen NN, DeSouza SV, Pendharkar SA, Petrov MS. Comprehensive analysis of body composition and insulin traits associated with intra‐pancreatic fat deposition in healthy individuals and people with new‐onset prediabetes/diabetes after acute pancreatitis. Diabetes Obes Metab. 2019;21(2):417‐423. doi: 10.1111/dom.13523 [DOI] [PubMed] [Google Scholar]
- 22. Caijing H, Xiaoting K, Xiaohong X, et al. Effects of ginseng peptides on the hypoglycemic activity and gut microbiota of a type 2 diabetes mellitus mice model. J Funct Foods. 2023;111:105897. doi: 10.1016/J.JFF.2023.105897 [DOI] [Google Scholar]
- 23. Mingjun Y, Jinhui L, Jumei Y, et al. Effects of Codonopsis pilosula crude polysaccharides by hypoglycemic and modulating gut microbiome in a high‐fat diet and streptozotocin‐induced mouse model of T2DM. J Funct Foods. 2023;111:105893. doi: 10.1016/J.JFF.2023.105893 [DOI] [Google Scholar]
- 24. Xiang Y, Cao Y‐N, Yang S‐H, et al. Isolation and purification of Tartary buckwheat polysaccharides and their effect on gut microbiota. Food Sci Nutr. 2023;11(1):408‐417. doi: 10.1002/fsn3.3072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang H‐R, Chen L‐H, Zeng Y‐J. Structure, antioxidant activity and in vitro hypoglycemic activity of a polysaccharide purified from Tricholoma matsutake . Foods. 2021;10(9):2184. doi: 10.3390/foods10092184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Duan W‐X, Yang X‐H, Zhang H‐F, Feng J, Zhang M‐Y. Chemical structure, hypoglycemic activity, and mechanism of action of selenium polysaccharides. Biol Trace Elem Res. 2022;200(10):4404‐4418. doi: 10.1007/s12011-021-03035-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhu Y, Yang L, Zhang C, Tian Y, Zhang F, Li X. Structural and functional analyses of three purified polysaccharides isolated from Chinese Huaishan‐yams. Int J Biol Macromol. 2018;120:693‐701. doi: 10.1016/j.ijbiomac.2018.08.143 [DOI] [PubMed] [Google Scholar]
- 28. Grewal AS, Kharb R, Prasad DN, Dua JS, Lather V. Design, synthesis and evaluation of novel 3,5‐disubstituted benzamide derivatives as allosteric glucokinase activators. BMC Chem. 2019;13(1):2. doi: 10.1186/s13065-019-0532-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Deng N, Zheng B, Li T, Liu RH. Assessment of the phenolic profiles, hypoglycemic activity, and molecular mechanism of different highland barley (Hordeum vulgare L.) varieties. Int J Mol Sci. 2020;21(4):1175. doi: 10.3390/ijms21041175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tiong SH, Looi CY, Arya A, et al. Vindogentianine, a hypoglycemic alkaloid from Catharanthus roseus (L.) G. Don (Apocynaceae). Fitoterapia. 2015;102:182‐188. doi: 10.1016/j.fitote.2015.01.019 [DOI] [PubMed] [Google Scholar]
- 31. Gong P, Long H, Guo Y, Wang S, Chen F, Chen X. Isolation, structural characterization, and hypoglycemic activities in vitro of polysaccharides from Pleurotus eryngii . Molecules. 2022;27(20):7140. doi: 10.3390/molecules27207140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Feng W, Liu Y, Fei F, et al. Improvement of high‐glucose and insulin resistance of chromium malate in 3T3‐L1 adipocytes by glucose uptake and insulin sensitivity signaling pathways and its mechanism. RSC Adv. 2019;9(1):114‐127. doi: 10.1039/c8ra07470d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang H, Shi S, Bao B, Li X, Wang S. Structure characterization of an arabinogalactan from green tea and its anti‐diabetic effect. Carbohydr Polym. 2015;124:98‐108. doi: 10.1016/j.carbpol.2015.01.070 [DOI] [PubMed] [Google Scholar]
- 34. Xu D‐q, Li C‐j, Jiang Z‐z, et al. The hypoglycemic mechanism of catalpol involves increased AMPK‐mediated mitochondrial biogenesis. Acta Pharmacol Sin. 2020;41(6):791‐799. doi: 10.1038/s41401-019-0345-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xiaoxia C, Chun C, Xiong F. Hypoglycemic activity in vitro and vivo of a water‐soluble polysaccharide from Astragalus membranaceus . Food Funct. 2022;13(21):11210‐11222. doi: 10.1039/D2FO02298B [DOI] [PubMed] [Google Scholar]
- 36. Jia Q, Liu X, Wu X, et al. Hypoglycemic activity of a polyphenolic oligomer‐rich extract of Cinnamomum parthenoxylon bark in normal and streptozotocin‐induced diabetic rats. Phytomedicine. 2008;16(8):744‐750. doi: 10.1016/j.phymed.2008.12.012 [DOI] [PubMed] [Google Scholar]
- 37. Zhou P, Xie W, He S, et al. Ginsenoside Rb1 as an anti‐diabetic agent and its underlying mechanism analysis. Cells. 2019;8(3):204. doi: 10.3390/cells8030204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zou S, Zhang X, Yao W, Niu Y, Gao X. Structure characterization and hypoglycemic activity of a polysaccharide isolated from the fruit of Lycium barbarum L. Carbohydr Polym. 2010;80(4):1161‐1167. doi: 10.1016/j.carbpol.2010.01.038 [DOI] [Google Scholar]
- 39. Perfumi M, Tacconi NAR. Hypoglycemic activity of Salvia fruticosa Mill. from Cyprus. J Ethnopharmacol. 1991;34(2–3):135‐140. doi: 10.1016/0378-8741(91)90030-h [DOI] [PubMed] [Google Scholar]
- 40. Ruxue Z, Jinhuang Z, Zhengping J, Yongxiang Z, Guoming G. Hypoglycemic effect of Rehmannia glutinosa oligosaccharide in hyperglycemic and alloxan‐induced diabetic rats and its mechanism. J Ethnopharmacol. 2004;90(1):39‐43. doi: 10.1016/j.jep.2003.09.018 [DOI] [PubMed] [Google Scholar]
- 41. Guo Q, Chen Z, Santhanam RK, et al. Hypoglycemic effects of polysaccharides from corn silk (Maydis stigma) and their beneficial roles via regulating the PI3K/Akt signaling pathway in L6 skeletal muscle myotubes. Int J Biol Macromol. 2018;121:981‐988. doi: 10.1016/j.ijbiomac.2018.10.100 [DOI] [PubMed] [Google Scholar]
- 42. Juncheng C, Liting W, Qingsong Z, et al. Structural characterization and in vitro hypoglycaemic activity of glucomannan from Anemarrhena asphodeloides Bunge. Food Funct. 2022;13(4):1797‐1807. doi: 10.1039/d1fo03010h [DOI] [PubMed] [Google Scholar]
- 43. Zhang Y, He Z, Liu X, et al. Oral administration of Angelica sinensis polysaccharide protects against pancreatic islets failure in type 2 diabetic mice: pancreatic β‐cell apoptosis inhibition. J Funct Foods. 2019;54:361‐370. doi: 10.1016/j.jff.2019.01.037 [DOI] [Google Scholar]
- 44. Feng‐Lin H, Min L I, Daih‐Huang K, Wang‐Chuan C, Hui‐Chen S, Juei‐Tang C. Antihyperglycemic effect of puerarin in streptozotocin‐induced diabetic rats. J Nat Prod. 2003;66(6):788‐792. doi: 10.1021/np0203887 [DOI] [PubMed] [Google Scholar]
- 45. Chen Y, Qi L, Zhong F, Li Y, Ke W, Ma Y. Integrated metabolomics and ligand fishing approaches to screen the hypoglycemic ingredients from four Coptis medicines. J Pharm Biomed Anal. 2020;192:113655. doi: 10.1016/j.jpba.2020.113655 [DOI] [PubMed] [Google Scholar]
- 46. Qian L, Wenzhi L, Qunyu G, Yuxiao Z. Hypoglycemic effect of Chinese yam (Dioscorea opposita rhizoma) polysaccharide in different structure and molecular weight. J Food Sci. 2017;82(10):2487‐2494. doi: 10.1111/1750-3841.13919 [DOI] [PubMed] [Google Scholar]
- 47. Deng Y, He K, Ye X, et al. Saponin rich fractions from Polygonatum odoratum (Mill.) Druce with more potential hypoglycemic effects. J Ethnopharmacol. 2012;141(1):228‐233. doi: 10.1016/j.jep.2012.02.023 [DOI] [PubMed] [Google Scholar]
- 48. Tzu‐Hsuan L, Chia‐Chung H, Lee‐Tian CC, Wen‐Chin Y. Anti‐hyperglycemic properties of crude extract and triterpenes from Poria cocos . Evid Based Complement Alternat Med. 2011;2011:128402. doi: 10.1155/2011/128402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Al‐Sultany FH, Al‐Hussaini IM, Saadi AHA. Studying hypoglycemic activity of Cuscuta chinesis Lam. on type 1 diabetes mellitus in white male rats. J Phys Conf Ser. 2019;1294(6):062020. doi: 10.1088/1742-6596/1294/6/062020 [DOI] [Google Scholar]
- 50. Konno C, Suzuki Y, Oishi K, Munakata E, Hikino H. Isolation and hypoglycemic activity of Atractans A, B and C, glycans of Atractylodes japonica rhizomes. Planta Med. 1985;51(2):102‐103. doi: 10.1055/s-2007-969418 [DOI] [PubMed] [Google Scholar]
- 51. Yitong W, Meixing Y, Ruiqing Q, Yanling G. Enzymolysis–microwave‐assisted hydrodistillation for extraction of volatile oil from atractylodes Chinensis and its hypoglycemic activity in vitro. J AOAC Int. 2021;104(4):1196‐1205. doi: 10.1093/jaoacint/qsab008 [DOI] [PubMed] [Google Scholar]
- 52. Gray AM, Flatt PR. Actions of the traditional anti‐diabetic plant, agrimony eupatoria (agrimony): effects on hyperglycaemia, cellular glucose metabolism and insulin secretion. Br J Nutr. 1998;80(1):109‐114. doi: 10.1017/s0007114598001834 [DOI] [PubMed] [Google Scholar]
- 53. Ezazul HM, Shahnaz R, Mohammed R, Rownak J. Evaluation of antihyperglycemic and antinociceptive activity of Xanthium indicum stem extract in Swiss albino mice. BMC Complement Altern Med. 2013;13(1):296. doi: 10.1186/1472-6882-13-296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Li Q, Qu H. Study on the hypoglycemic activities and metabolism of alcohol extract of Alismatis Rhizoma. Fitoterapia. 2012;83(6):1046‐1053. doi: 10.1016/j.fitote.2012.05.009 [DOI] [PubMed] [Google Scholar]
- 55. Jie Z, Jiguo H, Baoping J, Ye L, Xiaofeng Z. Antihyperglycemic effects of Platycodon grandiflorum (Jacq.) A. DC. extract on streptozotocin‐induced diabetic mice. Plant Foods Hum Nutr. 2007;62(1):7‐11. doi: 10.1007/s11130-006-0034-4 [DOI] [PubMed] [Google Scholar]
- 56. Kun Z, Mei H, Xia Z, et al. Hypoglycemic and antioxidant properties of extracts and fractions from Polygoni avicularis herba. Molecules. 2022;27(11):3381. doi: 10.3390/molecules27113381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Michael T, Isaac E, Richard M, Ronald KK, Wilson B. Hypoglycemic and toxic effect of Morus mesozygia leaf extract on the liver and kidneys of alloxan‐induced hyperglycemic Wistar rats. Evid Based Complement Alternat Med. 2019;2019:6712178. doi: 10.1155/2019/6712178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bilin X, Zhiliang L, Ting Z, et al. Bioactives of Momordica charantia as potential anti‐diabetic/hypoglycemic agents. Molecules. 2022;27(7):2175. doi: 10.3390/molecules27072175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Fei F, Xi‐rong W, Peng‐cheng Z. Research progress on hypoglycemic mechanism of traditional Chinese medicine compound preparations. J Chin Med Mater. 2011;34(6):996‐999. doi: 10.13863/j.issn1001-4454.2011.06.046 [DOI] [Google Scholar]
- 60. Qian W, Tao H, Jiali Z, et al. Integrated meta analysis and network pharmacology to investigate clinical evaluation and potential mechanism of Bushen Huoxue Decoction in treatment of diabetic nephropathy. Chin Tradit Herb Drugs. 2021;52(6):1692‐1705. [Google Scholar]
- 61. Chengxin S, Yan C, Xinzhi L, Guihua T, Yuying F, Yifa Z. Anti‐hyperglycemic and anti‐oxidative activities of ginseng polysaccharides in STZ‐induced diabetic mice. Food Funct. 2014;5(5):845‐848. doi: 10.1039/c3fo60326a [DOI] [PubMed] [Google Scholar]
- 62. Suzuki Y, Hikino H. Mechanisms of hypoglycemic activity of panaxans A and B, glycans of Panax ginseng roots: effects on the key enzymes of glucose metabolism in the liver of mice. Phytother Res. 1989;3(1):15‐19. doi: 10.1002/ptr.2650030105 [DOI] [Google Scholar]
- 63. Qingyu M, Ruohan Z, Xiaoqing X, et al. Hypoglycemic effects of Lycium barbarum polysaccharide in type 2 diabetes mellitus mice via modulating gut microbiota. Front Nutr. 2022;9:916271. doi: 10.3389/fnut.2022.916271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wu G, Bai Z, Wan Y, Shi H, Huang X, Nie S. Antidiabetic effects of polysaccharide from azuki bean (Vigna angularis) in type 2 diabetic rats via insulin/PI3K/AKT signaling pathway. Food Hydrocoll. 2020;101:105456. doi: 10.1016/j.foodhyd.2019.105456 [DOI] [Google Scholar]
- 65. Gong Y, Zhang J, Gao F, et al. Structure features and in vitro hypoglycemic activities of polysaccharides from different species of Maidong. Carbohydr Polym. 2017;173:215‐222. doi: 10.1016/j.carbpol.2017.05.076 [DOI] [PubMed] [Google Scholar]
- 66. Chen X, Jin J, Tang J, et al. Extraction, purification, characterization and hypoglycemic activity of a polysaccharide isolated from the root of Ophiopogon japonicus . Carbohydr Polym. 2010;83(2):749‐754. doi: 10.1016/j.carbpol.2010.08.050 [DOI] [Google Scholar]
- 67. Zhang Y, Ren C, Lu G, et al. Anti‐diabetic effect of mulberry leaf polysaccharide by inhibiting pancreatic islet cell apoptosis and ameliorating insulin secretory capacity in diabetic rats. Int Immunopharmacol. 2014;22(1):248‐257. doi: 10.1016/j.intimp.2014.06.039 [DOI] [PubMed] [Google Scholar]
- 68. Liu H, Qi X, Yu K, et al. AMPK activation is involved in hypoglycemic and hypolipidemic activities of mogroside‐rich extract from Siraitia grosvenorii (Swingle) fruits on high‐fat diet/streptozotocin‐induced diabetic mice. Food Funct. 2019;10(1):151‐162. doi: 10.1039/c8fo01486h [DOI] [PubMed] [Google Scholar]
- 69. Dai S, Hong Y, Xu J, Lin Y, Si Q, Gu X. Ginsenoside Rb2 promotes glucose metabolism and attenuates fat accumulation via AKT‐dependent mechanisms. Biomed Pharmacother. 2018;100:93‐100. doi: 10.1016/j.biopha.2018.01.111 [DOI] [PubMed] [Google Scholar]
- 70. Guo X, Sun W, Luo G, et al. Panax notoginseng saponins alleviate skeletal muscle insulin resistance by regulating the IRS1‐PI3K‐AKT signaling pathway and GLUT4 expression. FEBS Open Bio. 2019;9(5):1008‐1019. doi: 10.1002/2211-5463.12635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhang C, Qiao S, Wu J, et al. A new insulin‐sensitive enhancer from Silene viscidula, WPTS, treats type 2 diabetes by ameliorating insulin resistance, reducing dyslipidemia, and promoting proliferation of islet β cells. Pharmacol Res. 2021;165:105416. doi: 10.1016/j.phrs.2020.105416 [DOI] [PubMed] [Google Scholar]
- 72. Shujing A, Dou N, Ting W, et al. Total saponins isolated from Corni fructus via ultrasonic microwave‐assisted extraction attenuate diabetes in mice. Foods. 2021;10(3):670. doi: 10.3390/foods10030670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zhu M, Luo J, Lv H, Kong L. Determination of anti‐hyperglycaemic activity in steroidal glycoside rich fraction of lily bulbs and characterization of the chemical profiles by LC‐Q‐TOF‐MS/MS. J Funct Foods. 2014;6:585‐597. doi: 10.1016/j.jff.2013.12.002 [DOI] [Google Scholar]
- 74. Zhang L, Su S, Zhu Y, et al. Mulberry leaf active components alleviate type 2 diabetes and its liver and kidney injury in db/db mice through insulin receptor and TGF‐β /Smads signaling pathway. Biomed Pharmacother. 2019;112:108675. doi: 10.1016/j.biopha.2019.108675 [DOI] [PubMed] [Google Scholar]
- 75. Lu S‐S, Yu Y‐L, Zhu H‐J, et al. Berberine promotes glucagon‐like peptide‐1 (7‐36) amide secretion in streptozotocin‐induced diabetic rats. J Endocrinol. 2009;200(2):159‐165. doi: 10.1677/joe-08-0419 [DOI] [PubMed] [Google Scholar]
- 76. Patel MB, Mishra S. Hypoglycemic activity of alkaloidal fraction of Tinospora cordifolia . Phytomedicine. 2011;18(12):1045‐1052. doi: 10.1016/j.phymed.2011.05.006 [DOI] [PubMed] [Google Scholar]
- 77. Shu X‐S, Lv J‐H, Tao J, Li G‐M, Li H‐D, Ma N. Antihyperglycemic effects of total flavonoids from Polygonatum odoratum in STZ and alloxan‐induced diabetic rats. J Ethnopharmacol. 2009;124(3):539‐543. doi: 10.1016/j.jep.2009.05.006 [DOI] [PubMed] [Google Scholar]
- 78. de Santana Aquino DF, Monteiro TA, Lima Cardoso CA, et al. Investigation of the antioxidant and hypoglycemiant properties of Alibertia edulis (LC Rich.) AC Rich. leaves. J Ethnopharmacol. 2020;253:112648. doi: 10.1016/j.jep.2020.112648 [DOI] [PubMed] [Google Scholar]
- 79. Tu L, Wang R, Fang Z, et al. Assessment of the hypoglycemic and hypolipidemic activity of flavonoid‐rich extract from Angelica keiskei. Molecules. 2022;27(19):6625. doi: 10.3390/molecules27196625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wu F, Jin Z, Jin J. Hypoglycemic effects of glabridin, a polyphenolic flavonoid from licorice, in an animal model of diabetes mellitus. Mol Med Rep. 2013;7(4):1278‐1282. doi: 10.3892/mmr.2013.1330 [DOI] [PubMed] [Google Scholar]
- 81. Tanaka S, Han LK, Zheng YN, Okuda H. Effects of the flavonoid fraction from Ginkgo biloba extract on the postprandial blood glucose elevation in rats. Yakugaku Zasshi. 2004;124(9):605‐611. doi: 10.1248/yakushi.124.605 [DOI] [PubMed] [Google Scholar]
- 82. Jung UJ, Lee M‐K, Park YB, Kang MA, Choi M‐S. Effect of citrus flavonoids on lipid metabolism and glucose‐regulating enzyme mRNA levels in type‐2 diabetic mice. Int J Biochem Cell Biol. 2006;38(7):1134‐1145. doi: 10.1016/j.biocel.2005.12.002 [DOI] [PubMed] [Google Scholar]
- 83. Lin Z, Tong Y, Li N, Zhu Z, Li J. Network pharmacology‐based study of the mechanisms of action of anti‐diabetic triterpenoids from Cyclocarya paliurus . RSC Adv. 2020;10(61):37168‐37181. doi: 10.1039/d0ra06846b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Liu J, Yang J. Hypoglycemic effect of Forsythia suspensa leaves on diabetic mice. Agric Sci Technol. 2013;14(1):98‐99, 175. doi: 10.16175/j.cnki.1009-4229.2013.01.038 [DOI] [Google Scholar]
- 85. Kako M, Miura T, Nishiyama Y, Ichimaru M, Moriyasu M, Kato A. Hypoglycemic activity of some triterpenoid glycosides. J Nat Prod. 1997;60(6):604‐605. doi: 10.1021/np9605403 [DOI] [PubMed] [Google Scholar]
- 86. Bisio A, De Mieri M, Milella L, et al. Antibacterial and hypoglycemic diterpenoids from Salvia chamaedryoides . J Nat Prod. 2017;80(2):503‐514. doi: 10.1021/acs.jnatprod.6b01053 [DOI] [PubMed] [Google Scholar]
- 87. Jiang Z‐Y, Liu C‐J, Niu Q, et al. In vitro hypoglycemic diterpenoids from the roots of Croton yunnanensis . J Nat Prod. 2023;86:199‐208. doi: 10.1021/acs.jnatprod.2c00970 [DOI] [PubMed] [Google Scholar]
- 88. Wang J, Ha TKQ, Shi Y‐P, Oh WK, Yang J‐L. Hypoglycemic triterpenes from Gynostemma pentaphyllum . Phytochemistry. 2018;155:171‐181. doi: 10.1016/j.phytochem.2018.08.008 [DOI] [PubMed] [Google Scholar]
- 89. Liu XJ, Hu XK, Yang H, et al. A review of traditional Chinese medicine on treatment of diabetic nephropathy and the involved mechanisms. Am J Chin Med. 2022;50(7):1739‐1779. doi: 10.1142/s0192415x22500744 [DOI] [PubMed] [Google Scholar]
- 90. Wang J, Ma Q, Li Y, et al. Research progress on traditional Chinese medicine syndromes of diabetes mellitus. Biomed Pharmacother. 2020;121:109565. doi: 10.1016/j.biopha.2019.109565 [DOI] [PubMed] [Google Scholar]
- 91. Lu G, Yong Z, Dan L. Research progress on pathogenesis and drugs of type 2 diabetes mellitus. Chin J Clin Ration Drug Use. 2024;17(11):176‐180. doi: 10.15887/j.cnki.13-1389/r.2024.11.051 [DOI] [Google Scholar]
- 92. Huifen Z, Yuging Z, Qianying W, Wanrong Y, Huang H, Shuling H. Effect and safety of Huangqi Xiaoji Huazhuo decoction in treating patients with prediabetes mellitus of spleen‐deficiency phlegm‐dampness syndrome. Guiding J Tradit Chin Med Pharm. 2023;29(12):53‐56, 83. doi: 10.13862/j.cn43-1446/r.2023.12.011 [DOI] [Google Scholar]
- 93. Mengzhu G, Jindong Z, Zhaohui F, Yi Z, Di Y. Meta‐analysis of the efficacy and safety of Jinlida granules combined with metformin in the treatment of type 2 diabetes mellitus. Chin J Difficult Complicated Cases. 2023;22(11):1204‐1209. [Google Scholar]
- 94. Bairong X, Jing T, Dong N, Zuoying X, Boyong Q, Yongxia W. Efficacy and safety of compound danshen dripping pills combined with conventional western medicine in the treatment of coronary heart disease complicated with diabetes mellitus: a meta analysis. Tradit Chin Drug Res Clin Pharmacol. 2024;35(2):280‐290. doi: 10.19378/j.issn.1003-9783.2024.02.016 [DOI] [Google Scholar]
- 95. Ruijun SCW. Evaluation of clinical efficacy of Yiben Huoxue decoction in the treatment of elderly patients with diabetes mellitus. Diabetes New World. 2023;26(21):16‐19. doi: 10.16658/j.cnki.1672-4062.2023.21.016 [DOI] [Google Scholar]


