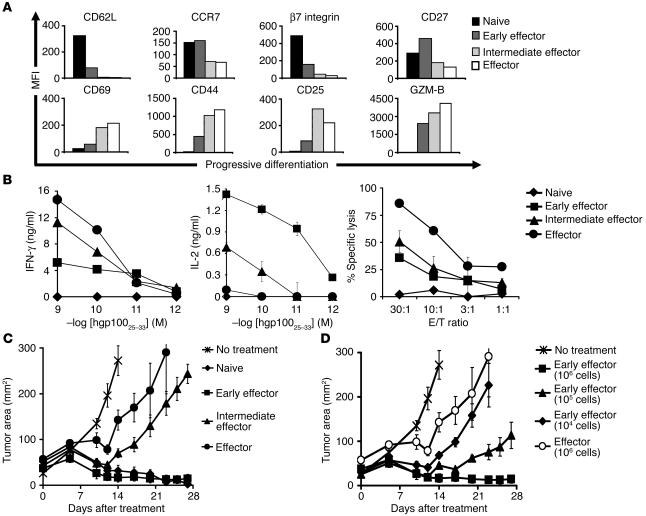
Figure 1.
Acquisition of full effector function in vitro impairs antitumor efficacy. Pmel-1 CD8+ T cells at progressive stages of differentiation were generated using multiple in vitro stimulations with 1 μM hgp10025–33 and 30 IU/ml of rhIL-2. Seven days following the last stimulation, phenotypic and functional assays were performed. (A) Phenotypic differences in naive and effector subgroups. Flow cytometry analysis for the expression of lymphoid-homing molecules CD62L, CCR7, and β7 integrin; costimulatory molecule CD27; activation/effector markers CD69, CD44, CD25, and granzyme-B (GZM-B) on CD8-enriched pmel-1 naive, early effector, intermediate effector, and effector T cells. Mean fluorescence intensity (MFI) values after gating on CD8+ cells are shown. Data shown are representative of 3 independent experiments. (B) Functional differences in naive and effector subgroups. IFN-γ and IL-2 release and cytotoxic assay against hgp10025–33–pulsed MCA-205 targets. MCA-205 cells plus the irrelevant influenza nucleoprotein peptide were used as control. In the cytotoxic assay, values for control target cells (0–14%) were subtracted. Data shown are representative of 3 independent experiments. (C and D) Differentiation of antitumor T cells is inversely associated with in vivo effectiveness. WT mice bearing 10-day-old established s.c. B16 tumors were sublethally irradiated and left untreated as control or received adoptive transfer of 1 × 106 (when not otherwise indicated) CD8-enriched pmel-1 naive, early effector, intermediate effector, and effector T cells in conjunction with rFPhgp100 vaccination and exogenous rhIL-2 (36 μg per dose). Results for tumor area are the mean of measurements from 5 mice per group (± SEM). Data shown are representative of 7 independent experiments.