Abstract
Objective
Evidence from low- and middle-income countries regarding the effect of smoking in people with diabetes is lacking. Here, we report the association of smoking with mortality in a large cohort of Mexican adults with diabetes.
Methods
Participants with diabetes mellitus (self-reported diagnosis, use of antidiabetic medications or HbA1c ≥ 6.5%) aged 35–74 years when recruited into the Mexico City Prospective Study were included. Cox regression confounder-adjusted mortality rate ratios (RRs) associated with baseline smoking status were estimated.
Results
Among 15,975 women and 8225 men aged 35–74 years with diabetes but no other comorbidities at recruitment, 2498 (16%) women and 2875 (35%) men reported former smoking and 2753 (17%) women, and 3796 (46%) men reported current smoking. During a median of 17 years of follow-up there were 5087 deaths at ages 35–74 years. Compared with never smoking, all-cause mortality RR was 1.08 (95%CI 1.01–1.17) for former smoking, 1.11 (95%CI 1.03–1.20) for current smoking, 1.09 (95%CI 0.99–1.20) for non-daily smoking, 1.06 (95%CI 0.96–1.16) for smoking < 10 cigarettes/day (median during follow-up 4 cigarettes/day), and 1.28 (95% CI 1.14–1.43) for smoking ≥ 10 cigarettes/day (median during follow-up 15 cigarettes/day). Mortality risk among daily smokers was greatest for COPD, lung cancer, cardiovascular diseases, and acute diabetic complications.
Conclusion
In this cohort of Mexican adults with diabetes, low-intensity daily smoking was associated with increased mortality, despite observing smoking patterns which are different from other populations, and over 5% of total deaths were associated with smoking.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12889-024-19536-0.
Keywords: Smoking, Diabetes, Mortality, Mexican population
Introduction
Diabetes mellitus is a significant public health problem, with over 530 million individuals living with the disease and around 6.7 million deaths attributable to diabetes worldwide in 2021 [1]. In Mexico, diabetes prevalence has tripled in the last three decades [2, 3] and currently affects one in six Mexican adults [1]. Besides the microvascular complications associated with this condition, diabetes is a well-documented accelerant of cardiovascular disease (CVD), which is the leading cause of premature mortality in Mexico. While comprehensive risk factor management to reduce CVD risk in people with diabetes is an integral aspect of diabetes care [4], addressing modifiable risk factors to reduce CVD burden remains a challenge in Mexico [2]. A key pervasive modifiable risk factor for diabetes and CVD incidence and mortality is tobacco smoking. Despite ratification of the WHO’s Framework Convention on Tobacco Control (FCTC) in 2004, prevalence of current smoking among Mexican adults has remained relatively stable in the past decade, with nearly 1 in every 5 Mexican adults reporting current smoking in 2016 [5]. While broader implementation of the FCTC is needed, characterizing mortality risk attributed to smoking in Mexican adults with diabetes could help inform tailored strategies for smoking cessation in this population.
The Mexico City Prospective Study (MCPS) is a large prospective study of 150,000 adults who were recruited between 1998 and 2004 and have been tracked for cause-specific mortality ever since. A previous analysis of the MCPS identified an increased risk of all-cause mortality among disease-free former and current smokers [6]. Risk of all-cause mortality was increased even among adults who reported low-intensity daily smoking. However, participants with diabetes and other chronic diseases were excluded to minimize reverse causality [6]. Despite the important contribution of this analysis to the literature on smoking and mortality, smoking patterns in people with diabetes as well as smoking-related mortality in Mexican adults with diabetes remains unexplored. In this study, we aim to characterize patterns of tobacco use in individuals with diabetes and to estimate the effect of smoking on all-cause and cause-specific mortality, exploring the influence of diabetes-related factors (glycaemic control and disease duration) as potential modifiers of this association.
Methods
Cohort characteristics
We analysed data from participants enrolled in the MCPS, a prospective, population-based cohort study with a baseline survey conducted from 1998 to 2004 [7]. Households from two urban districts of Mexico City (Coyoacán and Iztapalapa) were visited, and every adult ≥ 35 years was invited to participate. In total, 159,755 individuals were recruited, and information was collected regarding age, sex, lifestyle habits, self-reported medical history and comorbidities, self-reported use of medications, and socio-demographic information including education, civil status, occupation, and monthly income. Clinical measurements (weight, height, and blood pressure) were obtained, and a non-fasting 10-mL blood sample was collected for subsequent analysis. From 2015 to 2019, a follow-up survey was conducted on 10,143 surviving participants, obtaining similar data regarding lifestyle habits, medical history, clinical measurements, and another blood sample as described previously [8]. Blood samples, both at baseline and resurvey examinations, were stored overnight in a central laboratory, separated into plasma and buffy coat, and then frozen to − 80ºC. Afterwards, samples were shipped to the University of Oxford for analysis and storage. Assays of HbA1c were performed from buffy coat samples in the Clinical Trial Service Unit and Epidemiological Studies Unit’s Wolfson laboratory, which has International Organization for Standardization (ISO)-17,025 accreditation [9]. Buffy-coat samples can be used to obtain reliable assays of HbA1c [10], and these assays have been used in previous analyses of MCPS [8, 9, 11]. The study protocol was approved by the corresponding ethics committees at the Mexican Ministry of Health, the Mexican National Council for Science and Technology, and the University of Oxford. All study participants provided written informed consent.
Study population
We defined diabetes mellitus according to self-reported medical diagnosis of diabetes, use of diabetes medication (biguanides, sulfonylureas, insulin or other), or HbA1c ≥ 6.5% [12]. Diagnosed diabetes was defined as previous medical diagnosis of diabetes or use of glucose lowering medication and undiagnosed diabetes as HbA1c ≥ 6.5% in an individual without a previous diagnosis of diabetes. Given the heterogeneity of diabetes mellitus [13], and to better account for diabetes-related variables, the primary analysis was restricted to individuals with previously diagnosed or undiagnosed diabetes mellitus, excluding participants without diabetes. To address the possibility of reverse causality in the association between smoking and all-cause mortality, we excluded participants who reported comorbidities other than diabetes at baseline, including ischaemic heart disease, pulmonary disease, chronic kidney disease, cirrhosis, or cancer. We also excluded individuals with missing covariates or with uncertain mortality linkage.
Smoking related variables
We defined smoking status according to baseline interview information. Never smokers were classified as those who reported never smoking, former smokers as those who reported having smoked previously but not currently and current smokers as those having ever smoked and smoking currently (daily or non-daily). We classified smoking intensity into five categories (never smoker, ex-smoker, current non-daily smoker, current daily smoker < 10 cigarettes per day, and current daily smoker ≥ 10 cigarettes per day), and age when started smoking into three categories (< 15 years, 15–23 years, ≥ 24 years). In former smokers, no data was available regarding time since they stopped smoking; therefore, we were unable to explore this characteristic. We also analysed changes in smoking status between baseline interview and resurvey to explore changes in smoking behaviour over the study period. To do this, we matched surviving individuals who were included in the main mortality analysis at baseline and resurveyed during the 2015–2019 period. Then, we calculated the proportion of participants with a given smoking status at baseline and the proportion at resurvey.
Mortality follow-up
Mortality follow-up of participants was done through electronic probabilistic linkage to death registries, with deaths tracked up to December 31st, 2020, and registered according to the International Classification of Diseases 10th Revision (ICD-10). Cause-specific mortality was classified into several categories (cardiovascular, myocardial infarction, stroke, other vascular, acute diabetes complications [diabetic coma and ketoacidosis], lung cancer, non-lung cancer, COPD, other respiratory, renal, hepatobiliary, and gastric) in accordance with the underlying causes of death determined by study clinicians. The specific ICD-10 codes used for every cause and the number of deaths in each of them are provided in Supplementary Table 1.
Statistical analyses
For descriptive analyses, we calculated mean and standard deviation in continuous variables (median and interquartile range for HbA1c, number of cigarettes per day and age started smoking), and proportions for categorical variables. To explore the association between smoking, all-cause and cause-specific mortality amongst individuals with diabetes, we fitted Cox proportional hazard regression models to estimate adjusted hazard ratios (HR) for former and current smokers using individuals with diabetes who were never smokers as the reference group with analyses restricted to premature mortality (deaths at ages 35–74 years). All models were stratified by sex and age at risk (5-year age groups) [14] and adjusted for place of residence (Coyoacán or Iztapalapa), educational level (elementary, high school, university, other), body mass index (BMI), HbA1c, time since diabetes diagnosis (in years, as a continuous variable), and alcohol consumption (never, former, up to 3 times a month, up to 2 times a week, ≥ 3 times a week). We tested the proportional hazards assumption using Schoenfeld residuals. As sensitivity analyses, we fitted separate Cox models for males and females to further characterize whether there are different risk profiles by sex, as well as fitting the models with number of cigarettes/d categorized by quartiles of consumption. We estimated the fraction of deaths attributable to smoking as the number of deaths x (RR – 1) / RR in former and current smokers, divided by the total number of deaths [9, 15]. In the text, conventional 95% confidence intervals (CI) comparing two groups are used. In the figures, however, group specific 95% CIs, are shown for every RR (including the reference group); these were estimated using floating absolute risks to reflect the amount of information in each category [16]. All statistical analyses were conducted using R software version 4.2.1.
Results
Smoking status and baseline characteristics of participants with diabetes
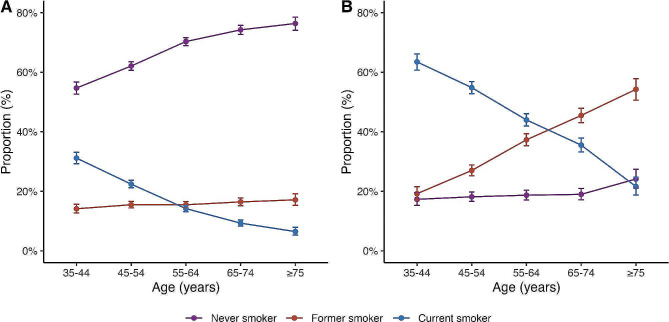
Among 159,755 participants recruited from 1998 to 2004, we identified 29,948 individuals with diabetes at baseline. We excluded 3,953 of these participants for having comorbidities other than diabetes, such as ischemic heart disease, pulmonary disease, chronic kidney disease, cirrhosis, or cancer. Additionally, 1,795 participants were excluded due to missing covariate or mortality data. In total, mortality analyses included 24,200 participants with diabetes who had complete data and no comorbidities. (Supplementary Fig. 1). Among women, 10,724 (67.1%) reported being never smokers, 2,498 (15.6%) former smokers, and 2,753 (17.2%) current smokers at baseline (Table 1). Among men, there was a lower proportion of never smokers with 1,554 (18.89%), and higher proportions of former and current smokers, with 2,875 (34.95%) and 3,796 (46.2%), respectively. Exploring smoking prevalence stratified by baseline age, the greatest proportion of female participants were never smokers and the proportion of current smokers decreased with age. Cessation, however, was uncommon in women with a consistent number of former smokers across all age cohorts (Fig. 1A). In men, on the other hand, there was a higher proportion of current smokers and the proportion of former smokers increased with age. The proportion of never smokers was relatively low regardless of age (Fig. 1B). Amongst current smokers, the prevalence of non-daily smoking was higher at younger ages for both men and women, low-intensity daily smoking (< 10 cig/day) increased for older individuals irrespective of sex, and high-intensity smoking remained stable throughout all age groups (Supplementary Fig. 2).
Table 1.
Baseline characteristics of participants with diabetes. Categorical variables presented as proportions. Continuous variables presented as mean and standard deviation for age, weight, height, BMI, systolic pressure, and diastolic pressure; and as median and interquartile range for age started smoking, number of cigarettes per day, and HbA1c. HbA1c: glycated haemoglobin, BMI: body mass index
| Women (n = 15,975) | Men (n = 8,225) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Current smokers (n = 2,753) | Current smokers (n = 3,796) | |||||||||
| Characteristics | Never smokers | Former smokers | Non-daily smokers | < 10 cigarettes/d | ≥ 10 cigarettes/d | Never smokers | Former smokers | Non-daily smokers | < 10 cigarettes/d | ≥ 10 cigarettes/d |
| No. of participants | 10,724 (67.13%) | 2498 (15.64%) | 1086 (6.8%) | 1294 (8.1%) | 373 (2.33%) | 1554 (18.89%) | 2875 (34.95%) | 1167 (14.19%) | 1648 (20.04%) | 981 (11.93%) |
| Mean age | 59.06 (± 11.88) | 58.26 (± 11.91) | 51.47 (± 10.32) | 53.02 (± 11.19) | 52.65 (± 10.35) | 58.62 (± 12.3) | 61.45 (± 11.67) | 52.98 (± 10.94) | 55.38 (± 11.21) | 54.62 (± 10.45) |
| Coyoacán (%) | 3112 (29.02%) | 827 (33.11%) | 329 (30.29%) | 430 (33.23%) | 123 (32.98%) | 514 (33.08%) | 1026 (35.69%) | 389 (33.33%) | 629 (38.17%) | 383 (39.04%) |
| University (%) | 327 (3.05%) | 151 (6.04%) | 64 (5.89%) | 94 (7.26%) | 35 (9.38%) | 254 (16.34%) | 410 (14.26%) | 193 (16.54%) | 217 (13.17%) | 184 (18.76%) |
| High school (%) | 1184 (11.04%) | 407 (16.29%) | 230 (21.18%) | 285 (22.02%) | 87 (23.32%) | 323 (20.79%) | 528 (18.37%) | 272 (23.31%) | 397 (24.09%) | 225 (22.94%) |
| Elementary (%) | 6270 (58.47%) | 1410 (56.45%) | 643 (59.21%) | 716 (55.33%) | 211 (56.57%) | 774 (49.81%) | 1521 (52.9%) | 608 (52.1%) | 858 (52.06%) | 460 (46.89%) |
| Other (%) | 2943 (27.44%) | 530 (21.22%) | 149 (13.72%) | 199 (15.38%) | 40 (10.72%) | 203 (13.06%) | 416 (14.47%) | 94 (8.05%) | 176 (10.68%) | 112 (11.42%) |
| Age started smoking | – | 20 (17, 30) | 22.5 (18, 32) | 20 (16, 29) | 18 (15, 24) | – | 16 (14, 20) | 18 (15, 20) | 17 (15, 20) | 16 (14, 19) |
| No. of cigarettes/d | – | 2 (1, 5) | 1 (1, 2) | 3 (2, 5) | 15 (10, 20) | – | 5 (2, 20) | 2 (1, 3) | 4 (2, 6) | 15 (10, 20) |
| Median HbA1c (%) | 8.37 (6.82, 10.66) | 8.46 (6.82, 10.75) | 8.83 (7, 11.03) | 8.74 (7, 10.84) | 8.37 (6.82, 10.93) | 8.46 (6.82, 10.48) | 8.1 (6.73, 10.29) | 8.83 (7, 10.75) | 8.65 (6.91, 10.75) | 9.01 (7, 10.84) |
| < 7 HbA1c (%) | 2923 (27.26%) | 688 (27.54%) | 271 (24.95%) | 323 (24.96%) | 112 (30.03%) | 442 (28.44%) | 914 (31.79%) | 287 (24.59%) | 435 (26.4%) | 236 (24.06%) |
| 7–10 HbA1c (%) | 4243 (39.57%) | 985 (39.43%) | 420 (38.67%) | 510 (39.41%) | 135 (36.19%) | 626 (40.28%) | 1157 (40.24%) | 461 (39.5%) | 639 (38.77%) | 386 (39.35%) |
| > 10 HbA1c (%) | 3558 (33.18%) | 825 (33.03%) | 395 (36.37%) | 461 (35.63%) | 126 (33.78%) | 486 (31.27%) | 804 (27.97%) | 419 (35.9%) | 574 (34.83%) | 359 (36.6%) |
| Weight (kg) | 67.28 (± 13.42) | 69.91 (± 14.38) | 71.37 (± 14.71) | 70.34 (± 14.79) | 72.53 (± 14.97) | 75.22 (± 13.41) | 75.38 (± 13.22) | 76.52 (± 13.95) | 74.98 (± 13.21) | 76.17 (± 14.24) |
| Height (cm) | 149.37 (± 6.17) | 150.47 (± 6.24) | 150.79 (± 6.3) | 151.85 (± 6.16) | 153.19 (± 6.59) | 162.84 (± 6.85) | 163.07 (± 6.93) | 163.44 (± 7) | 163.54 (± 6.81) | 165.42 (± 7.26) |
| BMI (kg/m2) | 30.11 (± 5.51) | 30.84 (± 5.87) | 31.38 (± 6.27) | 30.46 (± 5.89) | 30.9 (± 6.17) | 28.34 (± 4.58) | 28.32 (± 4.57) | 28.61 (± 4.9) | 28 (± 4.51) | 27.84 (± 5.28) |
| Systolic Pressure (mmHg) | 135.62 (± 18.87) | 135 (± 18.84) | 131.24 (± 18) | 131.42 (± 18.33) | 131.27 (± 17.43) | 133.75 (± 17.74) | 134.85 (± 18.38) | 131.63 (± 17.33) | 132.2 (± 17.43) | 131.46 (± 17.6) |
| Diastolic Pressure (mmHg) | 85.63 (± 11.14) | 85.79 (± 11.64) | 84.57 (± 10.93) | 83.73 (± 10.47) | 83.56 (± 11.16) | 86.12 (± 11.08) | 86.11 (± 11.17) | 85.48 (± 10.89) | 85.41 (± 11.15) | 84.99 (± 11.01) |
| Biguanides (%) | 1467 (13.68%) | 319 (12.77%) | 137 (12.62%) | 181 (13.99%) | 44 (11.8%) | 195 (12.55%) | 373 (12.97%) | 130 (11.14%) | 176 (10.68%) | 114 (11.62%) |
| Sulfonylureas (%) | 5441 (50.74%) | 1263 (50.56%) | 521 (47.97%) | 605 (46.75%) | 169 (45.31%) | 729 (46.91%) | 1501 (52.21%) | 541 (46.36%) | 788 (47.82%) | 453 (46.18%) |
| Insulin (%) | 564 (5.26%) | 175 (7.01%) | 53 (4.88%) | 80 (6.18%) | 21 (5.63%) | 74 (4.76%) | 115 (4%) | 44 (3.77%) | 63 (3.82%) | 34 (3.47%) |
Fig. 1.
Smoking prevalence by age and sex. (A) Smoking status by age in women, (B) Smoking status by age in men
Smoking status of participants with diabetes at resurvey
Smoking status changed significantly during follow-up. Among 10,143 participants resurveyed, we identified 1,246 individuals initially classified with diabetes (diagnosed and undiagnosed) whose data was used in the main mortality analyses. Of these surviving participants, 666 (53.5%) were never smokers, 239 (19.2%) were former smokers and 341 (27.4%) were current smokers at baseline. Among never smokers, most remained as such and < 1% started smoking during follow-up. Similarly, among former smokers, 71.5% remained as former smokers and only 3.7% restarted smoking (with the remaining 24.7% reporting never smoking at resurvey). However, changes in smoking status were most noticeable in current smokers. Over 59% reported quitting during the follow-up period, and only 28.4% continued smoking at resurvey. The median number of cigarettes smoked per day at resurvey among current smokers and former smokers was similar. At resurvey, those who were former smokers had better glycaemic control than current smokers (Table 2, Supplementary Fig. 3).
Table 2.
Changes in reported smoking habits from baseline to resurvey. Categorical variables presented as proportions. Continuous variables presented as median and interquartile range. HbA1c: glycated haemoglobin
| Smoking status at baseline | Other characteristics | ||||
|---|---|---|---|---|---|
| Smoking status at resurvey | Never smoker (n = 666) | Former smoker (n = 239) | Current smoker (n = 341) | HbA1c level at resurvey (%) | No. of cigarettes/d at resurvey |
| Never smoker | 622 (93.39%) | 59 (24.69%) | 40 (11.73%) | 7.73 (6.36, 9.38) | - |
| Former smoker | 41 (6.16%) | 171 (71.55%) | 204 (59.82%) | 7.64 (6.45, 9.56) | 3 (2, 8) |
| Current smoker | 3 (0.45%) | 9 (3.77%) | 97 (28.45%) | 8.19 (6.43, 9.61) | 3 (2, 10) |
All-cause mortality and smoking status among individuals with diabetes
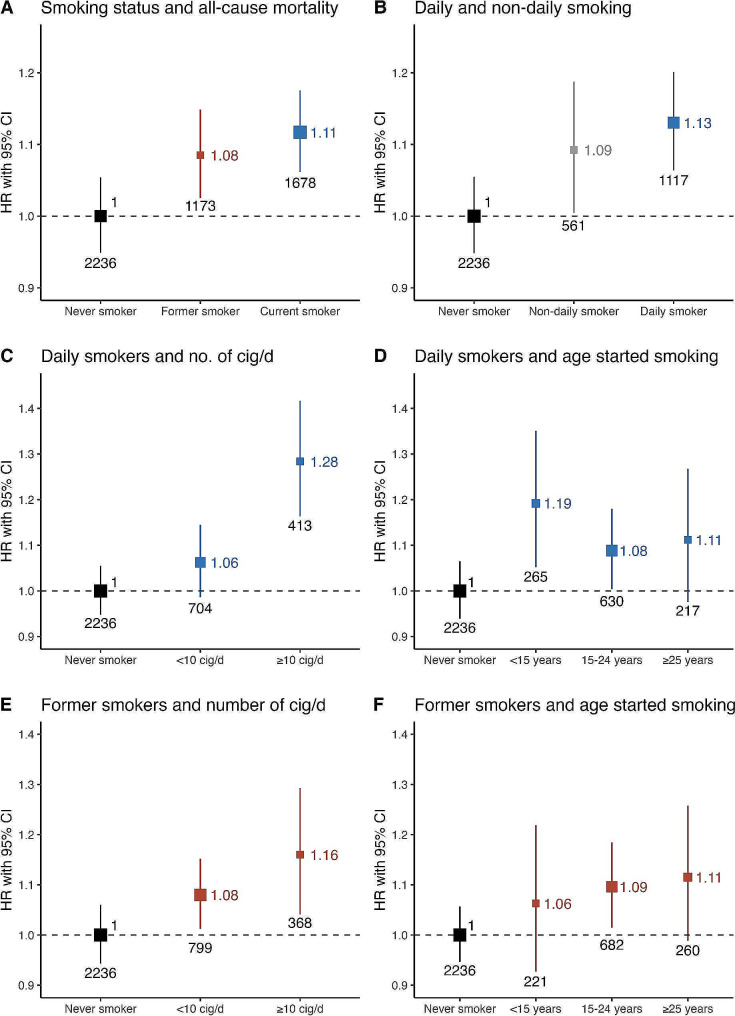
Among 24,200 participants with diabetes and after a median follow-up of 17.65 years, there were a total of 5,087 deaths at ages 35 to 74 years, 2,893 in women and 2,194 in men. Compared to never smokers, there was an increased risk for all-cause mortality among former (HR 1.08, 95%CI 1.01–1.17) and current smokers (HR 1.11, 95%CI 1.03–1.20, Fig. 2A), with similar relative risks in daily and non-daily smokers (Fig. 2B). Among daily smokers, there was increased risk in individuals who smoked ≥ 10 cigarettes/day (HR 1.28, 95%CI 1.14–1.43, Fig. 2C). There was similar mortality risk across age categories, being highest among those who started smoking < 15 years (HR 1.19, 95%CI 1.03–1.37, Fig. 2D). Exploring all-cause mortality risk in former smokers, we found increased risk for participants who previously smoked ≥ 10 cigarettes/day (HR 1.16, 95%CI 1.02–1.31) but similar RRs irrespective of age starting (Fig. 2E, F). Our results show an excess risk of death associated with smoking of 5% among individuals with diabetes aged 35–74 years, of which 1.8% was associated to former smoking and 3.4% to current smoking (Supplementary Table 2).
Fig. 2.
Hazard ratios for all-cause mortality by smoking status in individuals with diabetes. (A) Smoking status and all-cause mortality, (B) Non-daily, daily smoking and all-cause mortality in current smokers, (C) Number of cigarettes/day and all-cause mortality in daily smokers, (D) Age started smoking and all-cause mortality in daily smokers, (E) Number of cigarettes/day and all-cause mortality in former smokers, (F) Age started smoking and all-cause mortality in former smokers. All models were stratified by sex and age at risk (5-year age groups) and adjusted for place of residence (Coyoacán or Iztapalapa), educational level (elementary, high school, university, other), body mass index (BMI), HbA1c, time since diabetes diagnosis, and alcohol consumption
Smoking and cause-specific mortality among individuals with diabetes
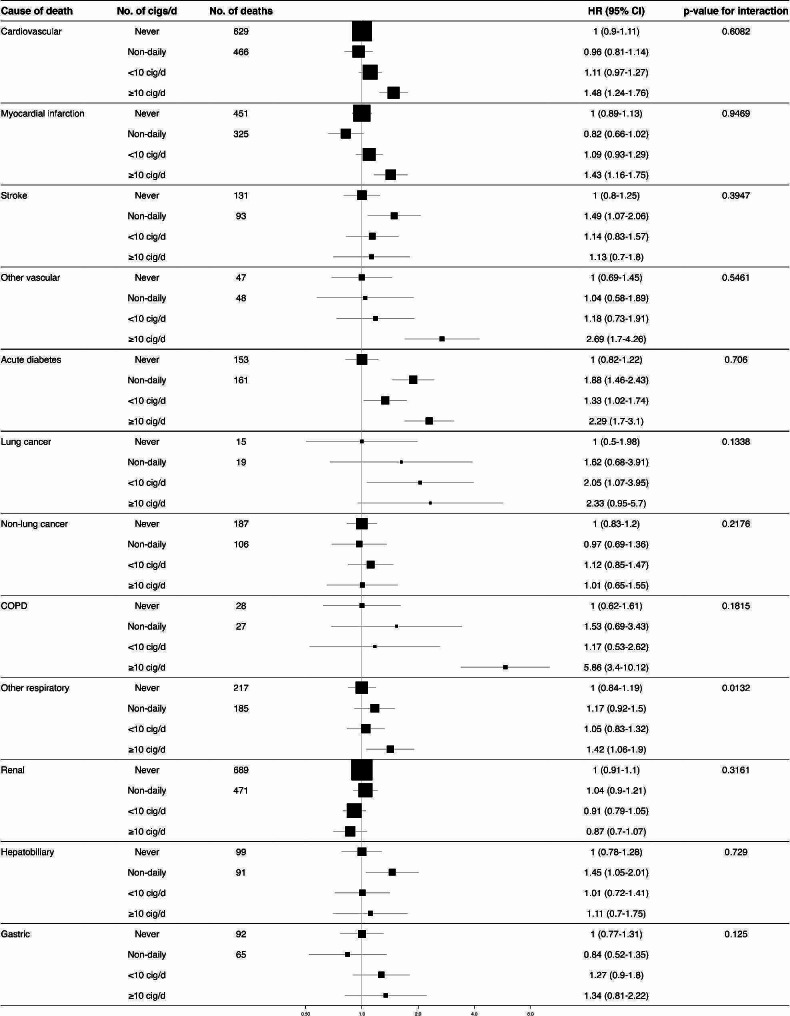
Compared to never smokers, daily smokers had a significantly higher risk of cardiovascular mortality especially in those smoking ≥ 10 cigarettes/day (HR 1.48 95%CI 1.24–1.76) (Fig. 3), with particularly high relative risks for myocardial infarction (HR 1.43 95%CI 1.16–1.75), and other vascular causes of death (HR 2.69 95%CI 1.70–4.26). There was no association between daily smoking and stroke mortality. However, we found an increased risk of stroke mortality among non-daily smokers (HR 1.49 95%CI 1.07–2.06). Interestingly, every smoking category was associated with mortality due to acute diabetes complications, including non-daily and former smokers (Supplementary Fig. 4). As expected, there were higher risks of death due to lung cancer for those smoking < 10 cigarettes per day (HR 2.05 95%CI 1.07–3.95), and due to COPD among former smokers (HR 2.04 95%CI 1.37–3.04), and participants smoking ≥ 10 cigarettes per day (HR 5.86 95%CI 3.40–10.12). Similarly, we found increased relative risks for hepatobiliary causes of death among former smokers (HR 1.41 95CI% 1.13–1.77), and non-daily smokers (HR 1.45 95CI% 1.05–2.01). There was no risk association between smoking and mortality due to renal causes, non-lung cancer, nor gastrointestinal deaths, although the power to detect an association was probably low in the latter categories.
Fig. 3.
Cause-specific mortality in individuals with diabetes according to number of cigarettes smoked per day amongst daily smokers. COPD: Chronic obstructive pulmonary disease. All models were stratified by sex and age at risk (5-year age groups) and adjusted for place of residence (Coyoacán or Iztapalapa), educational level (elementary, high school, university, other), body mass index (BMI), HbA1c, time since diabetes diagnosis, and alcohol consumption
Sensitivity analyses
We conducted a sensitivity analysis for all-cause mortality including individuals with comorbidities, obtaining similar estimations to those without comorbidities. Slightly higher point estimates were found in former smokers, which suggests that the presence of complications due to comorbidities may influence the effect of smoking in this subgroup of individuals (Supplementary Fig. 5). We also fitted Cox models with the number of cigarettes/day categorized into quartiles of consumption. Among daily smokers, there was a significant risk association among individuals in Q2 (HR 1.21, 95%CI 1.05–1.40), Q3 (HR 1.18, 95%CI 1.05–1.33), and Q4 (HR 1.17, 95%CI 1.02–1.35) compared to never smokers. Among non-daily smokers there was a risk association only among those in Q1 (HR 1.14, 95%CI 1.01–1.29), and among former smokers we found risk associations in every quartile except Q2 (Supplementary Fig. 6) Finally, we performed a sensitivity analysis to explore the association between mortality risk and smoking at ages ≥ 75 years, finding no significant mortality association at these ages (Supplementary Fig. 7).
Discussion
In this study of 24,200 participants with diabetes and no other comorbidities in the Mexico City Prospective Study, daily and non-daily smoking were associated with an increased risk for all-cause mortality in a dose-dependent manner. Daily smokers had higher mortality risk in those who smoked ≥ 10 cigarettes/day and in participants who started smoking aged < 15 years. This association remained in daily smokers, non-daily smokers, and former smokers even after categorizing cigarette consumption into quartiles compared to never smokers. Notably, over 50% of current smokers at baseline had stopped smoking at resurvey, which suggests a significant change in smoking habits during follow-up. Daily smokers were also at increased risk of death due to causes directly related to smoking (cardiovascular disease, myocardial infarction, other vascular disease, lung cancer and COPD), but also due to acute diabetes complications. Among former smokers with diabetes, a significant proportion remained as such at resurvey, with increased mortality risk in those who previously smoked ≥ 10 cigarettes/day and in those who started smoking at ages 15–24. Former smokers were at increased risk of death primarily due to COPD, hepatobiliary disease and, interestingly, acute diabetes complications as well. Interestingly, non-daily smokers also presented higher relative risks primarily for stroke, acute diabetes complications, and hepatobiliary causes of death. These findings confirm that smoking represents an excess risk that is modifiable in this population and support the notion that it remains a significant risk factor to be considered to reduce cardiovascular risk as part of long-term diabetes management.
The effect of smoking on all-cause and cause-specific mortality has been extensively reported in the general population [17, 18] and numerous studies have also explored the association between smoking and mortality risk in individuals with diabetes, finding a predominately positive association [19–21]. Regarding cause-specific mortality, smoking among individuals with diabetes has been associated with significantly increased risk for coronary heart disease, stroke, and peripheral arterial disease [22, 23]. Our findings in this subgroup of participants are in line to those reported previously for all-cause mortality, albeit with lower estimates for both former and daily smokers 22]; and our cause-specific mortality analysis identified similar risk associations, primarily with cardiovascular disease and myocardial infarction for heavy smokers, but not with stroke probably related to lack of statistical power in our study population. Interestingly, our results show a significant impact on death related to acute hyperglycaemic crises in former, non-daily, and daily smokers. Smoking is not considered a typical risk factor for the development of either diabetic ketoacidosis or hyperglycaemic hyperosmolar state; however concomitant illness such as myocardial infarction or cerebrovascular events can be precipitating causes (among others like poor adherence to treatment or infection) [24, 25]. Consequently, the observed association in our analysis might be due to poor baseline glycaemic control and the known association between smoking and cardiovascular events, which might lead to higher risk of developing a precipitating cause and increased risk of acute glycaemic crises mortality in smokers. Additionally, given that current smokers presented worse glycaemic control regardless of sex, this association might signal lack of lifestyle modification or medication adherence, which could predispose active smokers to higher risk of acute glycaemic crises. These findings should further strengthen the fact that smoking cessation represents a pivotal intervention to reduce mortality risk for individuals with diabetes, and current smokers as an important group that should be targeted with comprehensive management strategies.
Additionally, a risk association between smoking and hepatobiliary causes of death was also found, especially in former smokers and non-daily smokers. Diabetes mellitus represents a significant risk factor for the development of non-alcoholic fatty liver disease (NAFLD) [26], the occurrence of hepatic fibrosis, and non-alcoholic steatohepatitis (NASH) [27]. Significantly, smoking has also been associated to higher risk of developing NAFLD [28, 29]. Taken together, the high hepatobiliary mortality risks associated with smoking, signal the importance of not only promoting smoking cessation in this population, but also considering taking recent recommendations related to screening for NAFLD and NASH in individuals with diabetes [30].
In Mexico, a previous analysis of the MCPS on healthy individuals found that smoking was responsible for significantly higher mortality risk among active smokers with a dose-response relationship [6], representing the first prospective analysis of smoking-related mortality in Mexican population. The ratification of WHO’s FCTC in Mexico in 2004 was followed by the approval of the 2008 General Law on Tobacco Control which allowed for banning of cigarette advertising and graphic health warnings on cigarette’s packages [31]. Additionally, by 2011 a 68% tax was imposed on cigarette products [32]. All of these measures were associated with an important decrease on current smoking prevalence in Mexico from 2000 to 2010 [5]. However, despite the progress, prevalence increased slightly between 2011 and 2016, and it has stalled since then around 19% [5, 33]. Moreover, prevalence of previously diagnosed diabetes has increased dramatically in Mexico reaching 10.2% in 2021 [3]. Even though smoking cessation is advised for every person with diabetes, it has been suggested that individuals with chronic diseases, particularly younger persons, have higher prevalence of smoking [34, 35]. Notably, the smoking trends among individuals with diabetes in Mexico are largely unknown, and so is the mortality risk associated with smoking, thus the rational to explore tobacco use in this subgroup of participants. It should be noted as well that previous analyses of MCPS have found high mortality rates in individuals with diabetes, which accounts for at least a third of all deaths at ages 35–74 years [11]. Given this conditions, even if the smoking-related RRs found in this analysis are somewhat lower than previously reported in individuals with diabetes [22] and in disease-free participants [6], the smoking-related mortality risk for individuals with diabetes might be similar or even greater in this population. Finally, it should be highlighted that several interventions have proved effective to promote smoking cessation in individuals with diabetes [4, 36], which emphasizes the importance of improving public health policies directed towards smoking cessation.
Strengths and limitations
Our study has several strengths, including its prospective design, which allows to obtain reliable estimates of the association between mortality risk and smoking in individuals with diabetes in a middle-income country, the large sample size obtained from two urban districts in Mexico City, and the analysis of resurvey data to assess changes of smoking habits during follow-up. We recognize however some limitations which should be considered to adequately interpret our findings. First, medication use was self-reported and some participants with controlled HbA1c who did not report glucose-lowering treatment might not have been included as individuals with diabetes. Second, information regarding characterization of former smokers is incomplete, which did not allow us to estimate the effect of time since smoking cessation in all-cause mortality. Third, because resurvey information was collected only in a subset of surviving participants, we were unable to explore the impact of changes in smoking exposure during follow-up on mortality analyses for every individual and, consequently, our estimates using baseline interview information do not account for changes in smoking patterns over time, which might lead to a potential underestimation of RRs, biasing the results towards the null. Reverse causality might also be a concern, particularly for participants who did not self-report comorbidities but who may present subclinical or undiagnosed comorbid conditions. Diabetes diagnosis might change the smoking status of participants which might be of concern in former smokers and in participants with undiagnosed diabetes. Given that we have limited information regarding why and when former smokers quitted and the precise moment undiagnosed participants were diagnosed with diabetes, caution should be taken regarding our estimates in these categories. Finally, due to the observational nature of the study, we are unable to rule out residual confounding despite controlling for known modifiers of mortality risk in statistical analyses.
Conclusions and perspectives
In summary, our results highlight that smoking in individuals with diabetes still represents an important risk factor for all-cause and cause-specific mortality. Former smokers with diabetes are at increased risk dependent on previous smoking intensity, whilst daily smokers have a dose-dependent increase in mortality risk, particularly for individuals who started smoking at younger ages. Notably, smoking is associated with higher risk of acute diabetes complications, which support the view that smoking cessation is still a relevant target to improve diabetes management and that it should be strongly recommended during patient counselling.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This project was registered and approved by the Research Committee at Instituto Nacional de Geriatría, project number DI-PI-006/2020. CAFM is enrolled at the PECEM Program of the Faculty of Medicine at UNAM. DRG and CAFM are supported by CONACyT. The authors thank the participants for their willingness to take part in this prospective study 20 years ago. This research was conducted using Mexico City Prospective Study (MCPS) data obtained through an open-access data request (application number 2022-012). We want to thank Jonathan Emberson and Diego Aguilar-Ramirez for their very thoughtful comments and methodological support on this manuscript.
Author contributions
Establishing the cohort: JBC, PKM, JAD and RTC. Obtaining funding: JBC, PKM, JAD, RTC and OYBC. Data acquisition, analysis, or interpretation of data: DRG, OYBC, CAFM, PSC, ANL, MRBA, LFC, AVV, JPE, JPDS, AKG, PAV, PKM, RTC, JAD, JAS, NEAV. Drafting first version of manuscript: DRG, OYBC. Critical revision of the report for important intellectual content: All authors. All authors have seen and approved the final version and agreed to its publication. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Funding
This research was supported by Instituto Nacional de Geriatría in Mexico. The funding sources had no role in the design, conduct or analysis of the study or the decision to submit the manuscript for publication.
Data availability
Data from the Mexico City Prospective Study are available to bona fide researchers. For more details, the study’s Data and Sample Sharing policy may be downloaded (in English or Spanish) from https://www.ctsu.ox.ac.uk/research/mcps. Available study data can be examined in detail through the study’s Data Showcase, available at https://datashare.ndph.ox.ac.uk/mexico/. Code is available for reproducibility of results at https://github.com/oyaxbell/smoking_diabetes_mcps/.
Declarations
Ethics approval and consent to participate
This project was registered and approved by the Research and Ethics Committee at Instituto Nacional de Geriatría, project number DI-PI-006/2020.
Consent for publication
Not applicable. Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jesus Alegre-Díaz, Jacqueline A. Seiglie, Neftali Eduardo Antonio-Villa and Omar Yaxmehen Bello-Chavolla Joint Last authorship.
Contributor Information
Jesus Alegre-Díaz, Email: inypcjad@gmail.com.
Omar Yaxmehen Bello-Chavolla, Email: oyaxbell@yahoo.com.mx.
References
- 1.International Diabetes Federation. IDF Diabetes Atlas. 10th ed. Internatinonal Diabetes Federation; 2021.
- 2.Bello-Chavolla OY, Rojas-Martinez R, Aguilar-Salinas C, Hernández-Avila M. Epidemiology of diabetes mellitus in Mexico. Nutr Rev. 2017;75(suppl 1). 10.1093/nutrit/nuw030. [DOI] [PubMed]
- 3.Shamah-Levy T, Romero-Martínez M, Barrientos-Gutierrez T. Encuesta Nacional De Salud Y Nutrición 2021 Sobre Covid-19. Resultados Nacionales. Instituto Nacional de Salud Pública; 2022.
- 4.ElSayed NA, Aleppo G, Aroda VR, et al. 5. Facilitating Positive Health Behaviors and Well-being to Improve Health outcomes: standards of Care in Diabetes—2023. Diabetes Care. 2023;46(Supplement1):S68–96. 10.2337/dc23-S005. 10.2337/dc23-S005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zavala-Arciniega L, Reynales-Shigematsu LM, Levy DT, et al. Smoking trends in Mexico, 2002–2016: before and after the ratification of the WHO’s Framework Convention on Tobacco Control. Tob Control. 2020;29(6):687–91. 10.1136/tobaccocontrol-2019-055153. 10.1136/tobaccocontrol-2019-055153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomson B, Tapia-Conyer R, Lacey B, et al. Low-intensity daily smoking and cause-specific mortality in Mexico: prospective study of 150 000 adults. Int J Epidemiol. 2021;50(3):955–64. 10.1093/ije/dyab013. 10.1093/ije/dyab013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tapia-Conyer R, Kuri-Morales P, Alegre-Díaz J, et al. Cohort Profile: the Mexico City prospective study. Int J Epidemiol. 2006;35(2):243–9. 10.1093/ije/dyl042. 10.1093/ije/dyl042 [DOI] [PubMed] [Google Scholar]
- 8.Aguilar-Ramirez D, Alegre-Díaz J, Gnatiuc L, et al. Changes in the diagnosis and management of diabetes in Mexico City between 1998–2004 and 2015–2019. Diabetes Care. 2021;44(4):944–51. 10.2337/dc20-2276. 10.2337/dc20-2276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herrington WG, Alegre-Díaz J, Wade R, et al. Effect of diabetes duration and glycaemic control on 14-year cause-specific mortality in Mexican adults: a blood-based prospective cohort study. Lancet Diabetes Endocrinol. 2018;6(6):455–63. 10.1016/S2213-8587(18)30050-0. 10.1016/S2213-8587(18)30050-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Youngman LD, Clark S, Manley S, Peto R, Collins R. Reliable Measurement of Glycated Hemoglobin in Frozen Blood samples: implications for epidemiologic studies. Clin Chem. 2002;48(9):1627–9. 10.1093/clinchem/48.9.1627. 10.1093/clinchem/48.9.1627 [DOI] [PubMed] [Google Scholar]
- 11.Alegre-Díaz J, Herrington W, López-Cervantes M, et al. Diabetes and cause-specific mortality in Mexico City. N Engl J Med. 2016;375(20):1961–71. 10.1056/NEJMoa1605368. 10.1056/NEJMoa1605368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.ElSayed NA, Aleppo G, Aroda VR, et al. 2. Classification and diagnosis of diabetes: standards of Care in Diabetes—2023. Diabetes Care. 2023;46(Supplement1):S19–40. 10.2337/dc23-S002. 10.2337/dc23-S002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahlqvist E, Storm P, Käräjämäki A, et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018;6(5):361–9. 10.1016/S2213-8587(18)30051-2. 10.1016/S2213-8587(18)30051-2 [DOI] [PubMed] [Google Scholar]
- 14.Carstensen B. Epidemiology with R. Oxford University Press; 2021.
- 15.Lash TL, VanderWeele TJ, Haneuse S, Rothman KJ, editors. Modern Epidemiology. Fourth edition. Wolters Kluwer; 2021.
- 16.Plummer M. Improved estimates of floating absolute risk. Stat Med. 2004;23(1):93–104. 10.1002/sim.1485. 10.1002/sim.1485 [DOI] [PubMed] [Google Scholar]
- 17.Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328(7455):1519. 10.1136/bmj.38142.554479.AE. 10.1136/bmj.38142.554479.AE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jha P, Ramasundarahettige C, Landsman V, et al. 21st-Century hazards of smoking and benefits of Cessation in the United States. N Engl J Med. 2013;368(4):341–50. 10.1056/NEJMsa1211128. 10.1056/NEJMsa1211128 [DOI] [PubMed] [Google Scholar]
- 19.Al-Delaimy WK, Willett WC, Manson JE, Speizer FE, Hu FB. Smoking and mortality among women with type 2 diabetes. Diabetes Care. 2001;24(12):2043–8. 10.2337/diacare.24.12.2043. 10.2337/diacare.24.12.2043 [DOI] [PubMed] [Google Scholar]
- 20.Vazquez-Benitez G, Desai JR, Xu S, et al. Preventable Major Cardiovascular events Associated with uncontrolled glucose, blood pressure, and lipids and active smoking in adults with diabetes with and without Cardiovascular Disease: a contemporary analysis. Diabetes Care. 2015;38(5):905–12. 10.2337/dc14-1877. 10.2337/dc14-1877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rawshani A, Rawshani A, Franzén S, et al. Risk factors, mortality, and Cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2018;379(7):633–44. 10.1056/NEJMoa1800256. 10.1056/NEJMoa1800256 [DOI] [PubMed] [Google Scholar]
- 22.Pan A, Wang Y, Talaei M, Hu FB. Relation of Smoking with Total Mortality and Cardiovascular events among patients with diabetes Mellitus: a Meta-analysis and systematic review. Circulation. 2015;132(19):1795–804. 10.1161/CIRCULATIONAHA.115.017926. 10.1161/CIRCULATIONAHA.115.017926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qin R, Chen T, Lou Q, Yu D. Excess risk of mortality and cardiovascular events associated with smoking among patients with diabetes: Meta-analysis of observational prospective studies. Int J Cardiol. 2013;167(2):342–50. 10.1016/j.ijcard.2011.12.100. 10.1016/j.ijcard.2011.12.100 [DOI] [PubMed] [Google Scholar]
- 24.Umpierrez G, Korytkowski M. Diabetic emergencies — ketoacidosis, hyperglycaemic hyperosmolar state and hypoglycaemia. Nat Rev Endocrinol. 2016;12(4):222–32. 10.1038/nrendo.2016.15. 10.1038/nrendo.2016.15 [DOI] [PubMed] [Google Scholar]
- 25.Dhatariya KK, Glaser NS, Codner E, Umpierrez GE. Diabetic ketoacidosis. Nat Rev Dis Primer. 2020;6(1):40. 10.1038/s41572-020-0165-1. 10.1038/s41572-020-0165-1 [DOI] [PubMed] [Google Scholar]
- 26.Younossi ZM, Golabi P, De Avila L, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol. 2019;71(4):793–801. 10.1016/j.jhep.2019.06.021. 10.1016/j.jhep.2019.06.021 [DOI] [PubMed] [Google Scholar]
- 27.Barb D, Repetto EM, Stokes ME, Shankar SS, Cusi K. Type 2 diabetes mellitus increases the risk of hepatic fibrosis in individuals with obesity and nonalcoholic fatty liver disease. Obesity. 2021;29(11):1950–60. 10.1002/oby.23263. 10.1002/oby.23263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okamoto M, Miyake T, Kitai K et al. Cigarette smoking is a risk factor for the onset of fatty liver disease in nondrinkers: A longitudinal cohort study. Vinciguerra M, ed. PLOS ONE. 2018;13(4):e0195147. 10.1371/journal.pone.0195147. [DOI] [PMC free article] [PubMed]
- 29.Yuan S, Chen J, Li X, et al. Lifestyle and metabolic factors for nonalcoholic fatty liver disease: mendelian randomization study. Eur J Epidemiol. 2022;37(7):723–33. 10.1007/s10654-022-00868-3. 10.1007/s10654-022-00868-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanwal F, Shubrook JH, Younossi Z, et al. Preparing for the NASH Epidemic: a call to action. Diabetes Care. 2021;44(9):2162–72. 10.2337/dci21-0020. 10.2337/dci21-0020 [DOI] [PubMed] [Google Scholar]
- 31.Ley General para el Control del Tabaco. Published online 2008. https://www.diputados.gob.mx/LeyesBiblio/pdf/LGCT.pdf.
- 32.World Health Organization. WHO Report on the Global Tobacco Epidemic, 2023. World Health Organization; 2023.
- 33.Barrera-Núñez DA, López-Olmedo N, Zavala-Arciniega L, Barrientos-Gutiérrez I, Reynales-Shigematsu LM. Consumo De tabaco y uso de cigarro electrónico en adolescentes y adultos mexicanos. Ensanut Continua 2022. Salud Pública México. 2023;65:s65–74. 10.21149/14830. 10.21149/14830 [DOI] [PubMed] [Google Scholar]
- 34.Bae J. Differences in cigarette Use behaviors by Age at the time of diagnosis with diabetes from Young Adulthood to Adulthood: results from the National Longitudinal Study of Adolescent Health. J Prev Med Pub Health. 2013;46(5):249–60. 10.3961/jpmph.2013.46.5.249. 10.3961/jpmph.2013.46.5.249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stanton CA, Keith DR, Gaalema DE, et al. Trends in tobacco use among US adults with chronic health conditions: National Survey on Drug Use and Health 2005–2013. Prev Med. 2016;92:160–8. 10.1016/j.ypmed.2016.04.008. 10.1016/j.ypmed.2016.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tonstad S, Lawrence D. Varenicline in smokers with diabetes: a pooled analysis of 15 randomized, placebo-controlled studies of varenicline. J Diabetes Investig. 2017;8(1):93–100. 10.1111/jdi.12543. 10.1111/jdi.12543 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from the Mexico City Prospective Study are available to bona fide researchers. For more details, the study’s Data and Sample Sharing policy may be downloaded (in English or Spanish) from https://www.ctsu.ox.ac.uk/research/mcps. Available study data can be examined in detail through the study’s Data Showcase, available at https://datashare.ndph.ox.ac.uk/mexico/. Code is available for reproducibility of results at https://github.com/oyaxbell/smoking_diabetes_mcps/.