Figure 1.
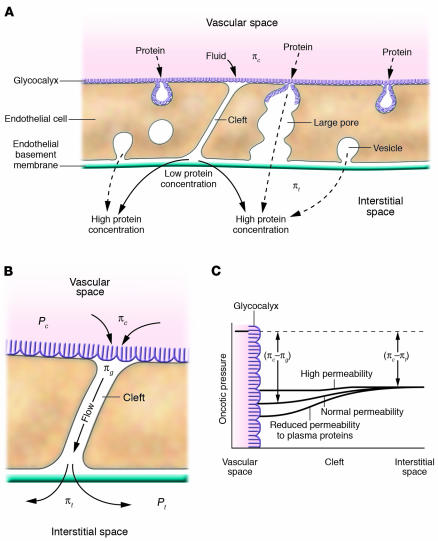
Pathways for water and plasma proteins across the endothelial barrier that may be regulated by ANP. (A) Pathways for plasma protein transport across the endothelial permeability barrier that ANP may modulate, including water and plasma transport through the interendothelial cleft at sites where there are infrequent breaks in the junctional strand (see refs. 1, 8) and specialized vesicular pathways and large pore pathways (1). The plasma protein concentration in the interstitial space is normally about 40% of that in the plasma. An ANP-induced increase in vascular permeability decreases the difference in plasma protein osmotic pressure between blood and the interstitial space and favors a reduced plasma volume. Conversely, decreased vascular permeability favors increased plasma volume. The latter mechanism would describe results of experiments where the ANP/BNP receptor, GC-A, on vascular endothelial cells is selectively deleted (6). Similar mechanisms are likely to act even more effectively when the endothelial glycocalyx has a low permeability to plasma proteins and contributes to the formation of a plasma ultrafiltrate with low_plasma protein concentration (and low-protein osmotic pressure). (B) Magnified view of the cleft pathway where the plasma protein concentration in the narrow space is lower than the mixed_plasma protein concentration in the interstitial space. Thus, as shown in C, the difference in plasma protein osmotic pressure across the glycocalyx (πc _ πg) may be significantly larger than the plasma-to-tissue colloid osmotic pressure (πc _ πt), and an ANP-induced increase in the permeability of the glycocalyx would favor a reduced plasma volume. πc, plasma protein osmotic pressure; πg, plasma protein osmotic pressure distal to the glycocalyx; πt, interstitial space protein osmotic pressure; Pc, capillary hydrostatic pressure; Pt, interstitial space hydrostatic pressure.