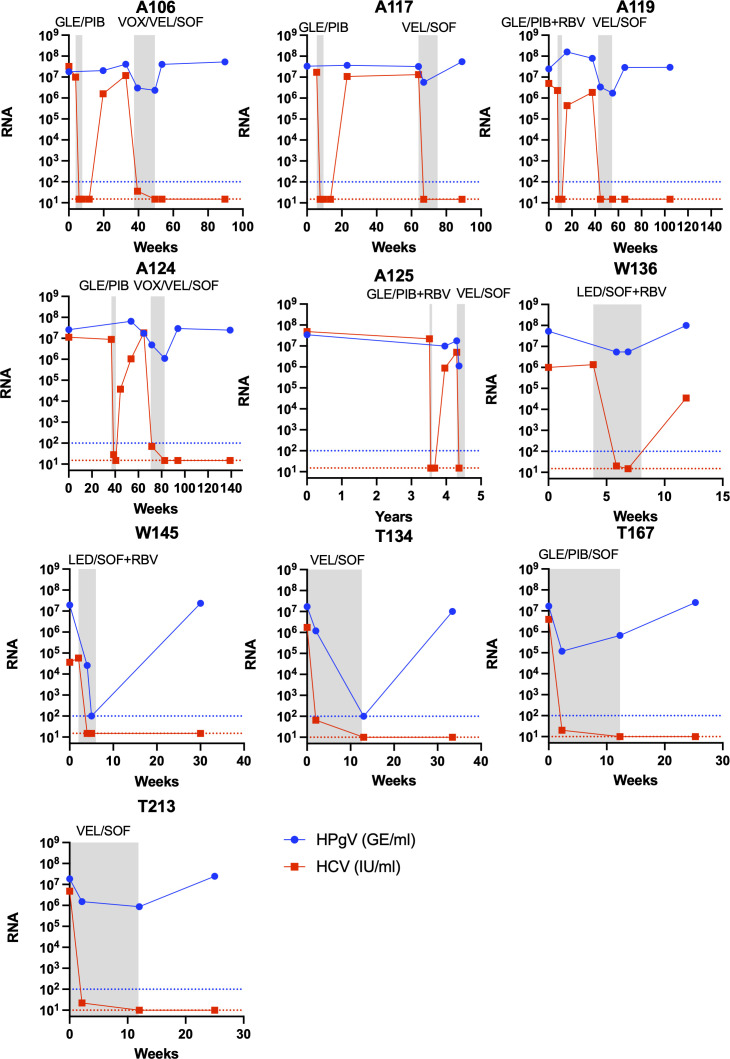
Fig 4.
RNA titer decrease of HCV and HPgV-1 in HCV patients during treatment with sofosbuvir-containing regimens. RNA titer measurements in plasma of HCV and HPgV-1 are shown at the y-axis as international units per milliliter (IU/mL) or genome equivalents per milliliter (GE/mL), respectively, and time at the x-axis. The gray boxes indicate periods during antiviral treatment with the type of treatment indicated at the top. Each panel represents a patient treated with regimens containing SOF, including LED/SOF, VEL/SOF, VOX/VEL/SOF, and GLE/PIB/SOF. A red titer line representing HCV RNA at the y-axis represents undetectable HCV RNA titers (limit of detection 15 IU/mL except for patients T134, T167, and T213 with limit of detection 10 IU/mL). Ribavirin addition to the treatment is indicated as RBV. A blue titer line representing HPgV-1 RNA at the y-axis represents undetectable HPgV-1 RNA titers (limit of detection 100 GE/mL).