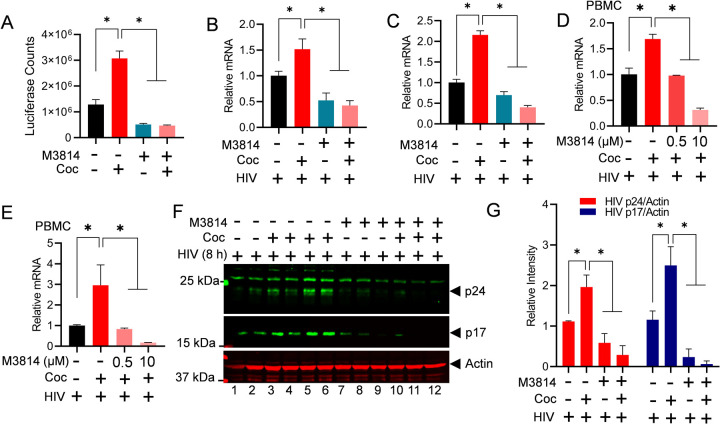
Figure 5: Cocaine-mediated DNA-PK activation promotes HIV transcription and replication in both cell lines and primary cells.
Jurkat-pHR’-P-Luc cells were treated with 10 µM of M3814 for 24 h. The next day, cells were treated with cocaine twice daily for 48 h and again 3 h before harvesting. Cells were lysed, and the level of reporter protein expression was determined using a luciferase reporter assay (A). Jurkat cells (B & C) and PBMCs (D & E) were treated with 10 µM of M3814 for 24 h, then treated with cocaine for 3 h, and subsequently infected with replication-competent HIV for another 3 to 6 h. HIV transcripts were quantified by real-time PCR using primer sets that amplify the Nuc-2 (B & D) and Env (C & E) regions of the HIV genome. Jurkat cells were treated with 10 µM of M3814 for 24 h (Lanes 7 to 12), then treated with cocaine for 3 h (Lanes 3–6 & 10–12) and infected with replication-competent HIV across all lanes (Lanes 1–12) for another 5 h. The levels of HIV p24 and p17 proteins were analyzed via immunoblotting using antibodies against these HIV proteins (F). Actin, a constitutively expressed protein, was used as a loading control. Densitometric analysis of protein bands (normalized to actin) was performed (G). Immunoblots are representative of at least three independent experiments. The results are expressed as mean ± SD and analyzed by two-way ANOVA followed by Tukey's multiple comparisons test. Asterisks over the bars indicate significant differences: ∗p < 0.05 for the comparison of cocaine-treated samples vs. untreated (Ctrl) and the comparison of cocaine plus inhibitor-treated samples vs. cocaine alone-treated samples.