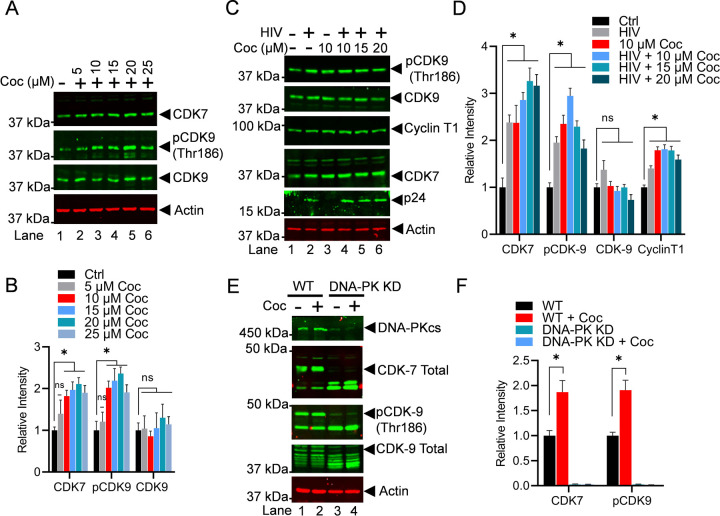
Figure 7: Cocaine enhances the elongation phase of HIV transcription not only by stimulating DNA-PK but also via P-TEFb activation.
Jurkat-pHR’P-Luc cells were treated with increasing doses of cocaine (5, 10, 15, 20, and 25 µM) for 3 h (A). Jurkat-pHR’P-Luc cells were treated as follows: untreated and uninfected (Lane 1), infected with HIV (93/TH/051) without cocaine treatment (Lane 2), treated with cocaine without HIV infection (Lane 3), or pre-treated with different concentrations of cocaine before HIV infection (Lanes 4 to 6) (C). Cells were harvested, and nuclear lysates were analyzed by immunoblotting with specific antibodies against P-TEFb subunits CDK9 and Cyclin T1, as well as CDK7 (TFIIH). Actin was used as a loading control. Densitometric analysis of protein bands (normalized to actin) confirmed a significant increase in CDK7, Cyclin T1, and p-CDK9 (Thr186) compared to untreated (Ctrl) cells (B & D). Wild type (WT) and DNA-PK knockdown (DNA-PK KD) cells were treated with cocaine for 30 min and 3 h, and nuclear extracts were subjected to immunoblotting (E). Densitometric analysis of protein bands (normalized to actin) showed increased p-CDK9 phosphorylation and CDK7 activation in WT cells upon cocaine exposure (F). However, in DNA-PK KD cells, the lack of p-CDK9 (Thr186) phosphorylation and CDK7 activation upon cocaine treatment demonstrated that cocaine-induced activations are DNA-PK specific (F). Immunoblots are representative of at least three independent experiments. The results are expressed as mean ± SD for three independent experiments, analyzed by two-way ANOVA followed by Tukey’s multiple comparisons test. Asterisks over the bars indicate significant differences: ∗p < 0.05 compared to untreated cells (Ctrl).