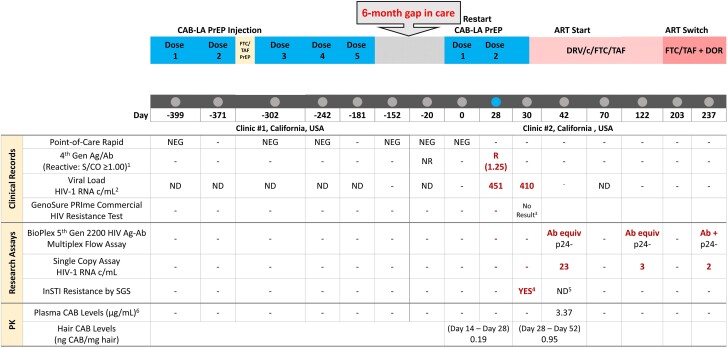
Figure 1.
Timeline of ARV administration and research and clinical testing for HIV diagnosis, PK, and ARV resistance. 1. ARCHITECT HIV Ag/Ab Combo; signal to cutoff value ≥1.00 considered reactive. 2. Clinic #1: Hologic Aptima HIV Quant Dx Assay, LOQ 30 copies/mL; Clinic #2: COBAS AmpliPrep/COBAS TaqMan HIV-1 Test, version 2.0; LOQ (limit of quantitation) 20 copies/mL. 3. No result due to failed PCR amplification in the commercial HIV genotyping assay. 4. Q148R was detected at 8% frequency and A128T was detected at 4% frequency on separate genomes. Full genotype is presented in Table 1. 5. Day 42 SGS limited in sensitivity to detect resistance due to HIV-1 RNA 23 copies/mL and only 3 genomes sequenced. 6. Plasma CAB concentration on day 42 (14 days after second injection) was ∼20× PA-IC90 (well above 8× PA-IC90). Abbreviations: Ag/Ab, antigen/antibody; ART, antiretroviral therapy; ARV, antiretroviral; CAB, cabotegravir; CAB-LA, long-acting cabotegravir; DOR, doravirine; DRV/c/FTC/TAF, darunavir/cobicistat/emtricitabine/tenofovir alafenamide; equiv, equivocal; FTC/TAF, emtricitabine/tenofovir alafenamide; INSTI, integrase strand transfer inhibitor; ND, not detected; NR, nonreactive; PK, pharmacokinetics; PrEP, pre-exposure prophylaxis; R, reactive; S/CO, signal to cutoff; SGS, single-genome sequencing.