Figure 2.
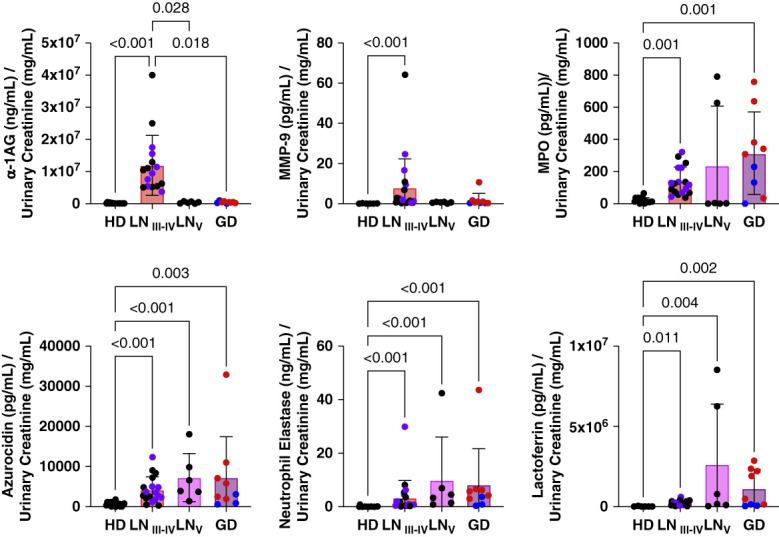
Enhanced urine excretion of granule proteins is associated with LN activity. Enhanced excretion of granule proteins were validated and compared in urine from patients with proliferative LN (LN 3–4), class 5 LN (LN 5), MCD, and primary MGN (other GD) and HDs by ELISA with normalization to urine creatinine concentrations. Azurophilic granule constituents measured included MPO (n=16 HD, n=18 LN 2–4, n=6 LN 5, n=9 GD), neutrophil elastase (n=16 HD, n=21 LN 2–4, n=6 LN 5, n=9 GD), and azurocidin (n=16 HD, n=18 LN 2–4, n=6 LN 5, n=9 GD). Specific granule constituents measured were α-1AG (n=15 HD, n=16 LN 2–4, n=6 LN 5, n=9 GD) and lactoferrin (n=6 HD, n=21 LN 2–4, n=6 LN 5, n=9 GD). The gelatinase granule constituent measured was MMP-9 (n=6 HD, n=21 LN I2–4, n=6 LN 5, n=9 GD). P values are shown for comparisons with statistical significance. MGN (red) and MCD (blue) results were include together as other GDs. In the proliferative LN group, purple represents patients with crescentic glomeruli on kidney biopsy. Enhanced granule protein excretion of granule proteins was validated in a separate patient cohort by ELISA with normalization to urine creatinine concentrations. The data were statistically analyzed using a series of Kruskal–Wallis tests with post hoc–corrected Dunn tests for multiple comparisons. Data were represented as mean±SD. GD, glomerular disease; HD, healthy donor; MCD, minimal change disease; MGN, membranous nephropathy.
