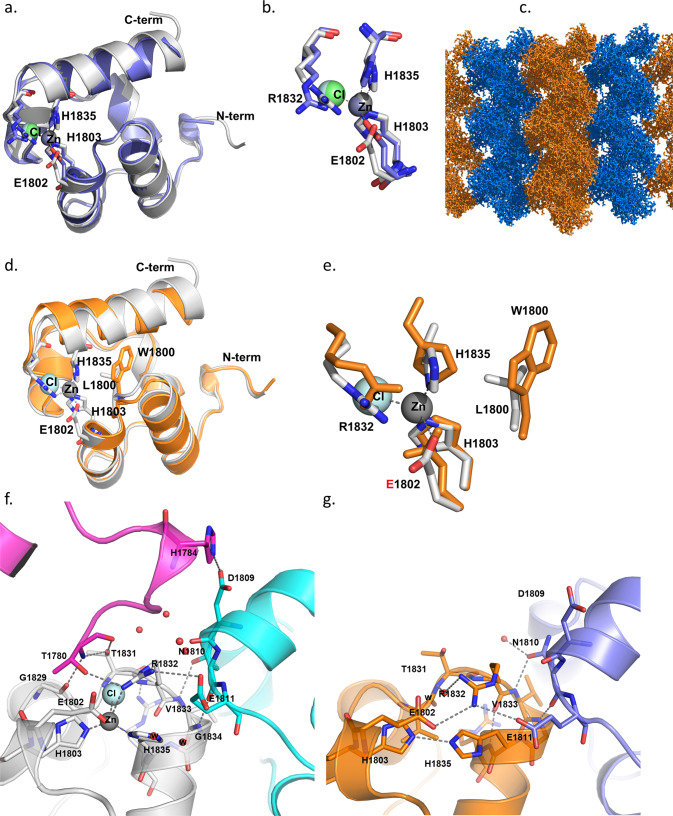
Fig. 3. Shank2-SAM binds Zn2+ and supports helical sheet formation.
a Overall fold representation of WT Shank2-SAM domain (colored in gray) aligned with Shank3-SAM domain (colored in blue) [13] (rmsd = 0.614 for superimposing 65 residues); b Zoom in representation of the Zn2+ cluster observed in both structures. In the Shank2-SAM structure, the Zn2+ ion only shows 30% occupancy and its tetrahedral coordination is distorted. The Cl− anion and E1802 are located at a distance longer than expected (2.9 Å and 3.2 Å, respectively), and R1832 is located closer (3.0 Å), establishing an interaction with the Cl− anion (3.2 Å). c Crystal packing representation of Shank2-SAM highlighting the observed helical sheets. d Overall fold representation of Shank2-SAM-WT domain (gray) aligned with Shank2-SAM-L1800W mutant domain (orange) (rmsd = 0.965 for superimposing 68 residues); e Zoom in representation of the Zn2+ cluster observed in Shank2-SAM-WT. The introduction of a bulkier side chain (W vs. L) in the mutant SAM domain induces a shift of the C-terminal helix (residues 1831:1849), moving H1835 (one of the Zn2+ coordinating residues) 2 Å away from its position in the SAM-WT structure. f, g Depiction of inter- and intra-fiber interactions in SAM-WT (f) and in SAM-L1800W (g). SAM-WT monomer is colored in gray, the symmetry related molecules are colored in magenta and cyan, respectively; SAM-L1800W monomer is colored in orange and the symmetry related molecule in blue. Note that there are intrahelical (cyan) and interhelical (magenta) SAM domains in the WT, but only an intrahelical domain for the mutant. Thus, the interhelical domain contact is lost in the mutant.