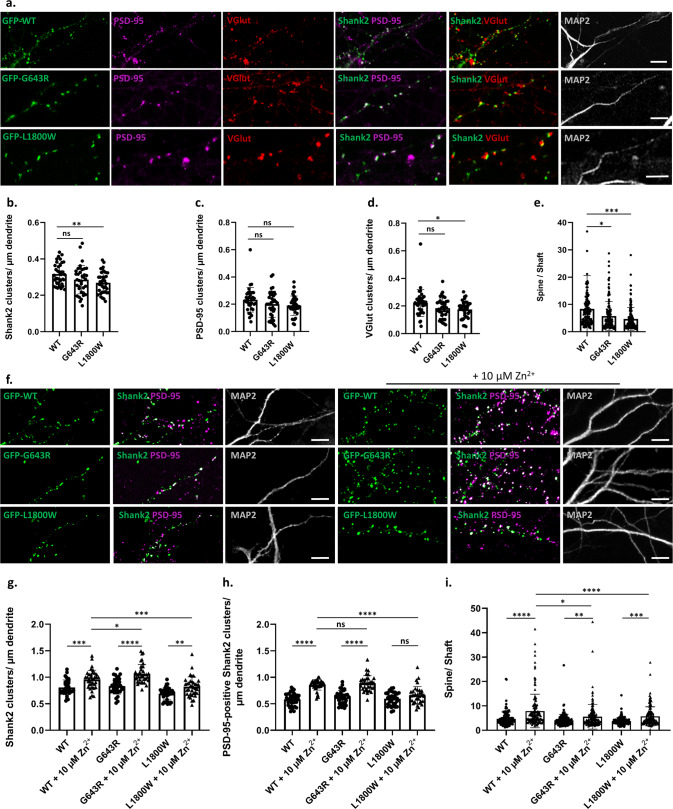
Fig. 4. Both mutant variants of Shank2 fail to properly reach postsynaptic sites.
a Primary cultured rat hippocampal neurons were co-transfected with an shRNA vector against the rat Shank2 mRNA, and an expression vector coding for GFP-tagged human Shank2 (WT or mutant, as indicated). Cells were stained with antibodies against the dendritic marker MAP2, the postsynaptic marker PSD-95, and the presynaptic marker VGlut. a, f Cells were analysed by confocal microscopy. Scale bar: 5 µm. b–d, g–h Quantitative analysis of at least 36 dendritic branches of 12–15 neurons obtained from three independent experiments. b The number of Shank2-positive clusters is decreased significantly upon expression of the L1800W mutant. c The number of PSD-95-positive clusters showed no difference among the three conditions. d The number of VGlut-positive clusters is reduced in neurons expressing the L1800W variant of Shank2. e, i Quantitative analysis of 140 clusters along dendrites of 12-15 neurons obtained from three independent experiments. The ratio of intensity of a GFP-positive dendritic cluster in relation to the intensity in the adjacent dendritic shaft was determined. e The spine/shaft signal intensity ratio was significantly reduced in both mutants when compared to the WT. f–h Cells were treated with a concentration of 10 µM Zn2+. g The number of Shank2 clusters is increased for all variants after Zn2+ treatment. h The number of PSD-95-positive Shank2 clusters is increased for WT and G643R variant after Zn2+ treatment, but not for the L1800W mutant. i The spine/shaft signal intensity ratio was significantly increased for all three conditions after Zn2+ treatment. *, **, ***, ****: significantly different, p < 0.05, 0.01, 0.001, 0.0001 respectively; two-way ANOVA, followed by both Sidak’s and Dunnett’s multiple comparison test.