Abstract
Poly (ADP-ribose) polymerase inhibitors (PARPi) can encounter resistance through various mechanisms, limiting their effectiveness. Our recent research showed that PARPi alone can induce drug resistance by promoting autophagy. Moreover, our studies have revealed that anaplastic lymphoma kinase (ALK) plays a role in regulating the survival of ovarian cancer cells undergoing autophagy. Here, we explored whether the ALK-inhibitor crizotinib could enhance the efficacy of PARPi by targeting drug-induced autophagic ovarian cancer cell and xenograft models. Our investigation demonstrates that crizotinib enhances the anti-tumor activity of PARPi across multiple ovarian cancer cells. Combination therapy with crizotinib and olaparib reduced cell viability and clonogenic growth in two-olaparib resistant cell lines. More importantly, this effect was consistently observed in patient-derived organoids. Furthermore, combined treatment with crizotinib and olaparib led to tumor regression in human ovarian xenograft models. Mechanistically, the combination resulted in increased levels of reactive oxygen species (ROS), induced DNA damage, and decreased the phosphorylation of AKT, mTOR, and ULK-1, contributing to increased olaparib-induced autophagy and apoptosis. Notably, pharmacologic, or genetic inhibition or autophagy reduced the sensitivity of ovarian cancer cell lines to olaparib and crizotinib treatment, underscoring the role of autophagy in cell death. Blocking ROS mitigated olaparib/crizotinib-induced autophagy and cell death while restoring levels of phosphorylated AKT, mTOR and ULK-1. These findings suggest that crizotinib can improve the therapeutic efficacy of olaparib by enhancing autophagy.
Implications: The combination of crizotinib and PARPi presents a promising strategy, that could provide a novel approach to enhance outcomes for patients with ovarian cancer.
Introduction
Ovarian cancer is the leading cause of gynecologic cancer mortality among women in the United States with a 5-year survival of approximately 30% (1). Poly ADP ribose polymerase (PARP) inhibitors (PARPi) have exerted significant activity against human ovarian cancers (2). PARP contributes to DNA damage repair through several different pathways, including homologous recombination (HR; ref. 3). In cancer cells with Homologous Recombination deficiency (HRD), PARP inhibition results in the accumulation of DNA double-strand breaks, ultimately leading to cell death. Maintenance treatment with PARPi including olaparib, rucaparib, and niraparib, have extended progression-free survival (PFS) for patients with a response to platinum-based chemotherapy in both the first line and recurrent settings (4). However, innate and acquired drug resistance often develop, limiting the efficacy of these agents. Inhibition of autophagy has been shown to increase the sensitivity of olaparib in ovarian cancer (5).
By using small-interfering RNA (siRNA) screens, knockdown of anaplastic lymphoma kinase (ALK) reduced the survival of autophagic ovarian cancer cells (6). Crizotinib, an anaplastic lymphoma kinase (ALK) inhibitor, decreases the viability of autophagic ovarian cancer cells in culture and improves survival in ovarian cancer xenografts with dormant autophagic DIRAS3-expressing cancer cells (6). Autophagy is a catabolic process that sequesters and degrades long lived proteins and damaged organelles, providing energy to cells under stress and under nutrient poor conditions (7). Induction of autophagy can be triggered by various factors including chemotherapy, radiation therapy, and targeted therapies. Autophagy can either protect cancer cells from chemotherapy or enhance their response to certain anti-cancer therapies (8, 9).
In our current study, we asked whether crizotinib could enhance the antitumor activity of olaparib and other PARPi in ovarian cancers. We have found that crizotinib enhances response to olaparib in a panel of human ovarian cancer cell lines, patient-derived organoids, and xenograft models. Crizotinib treatment also further enhanced olaparib-induced autophagy and promoted cell death by enhancing ROS formation and decreasing phosphorylation of AKT, mTOR and ULK-1(Ser757). Since PARPi and crizotinib are FDA approved anti-cancer drugs, PARPi/crizotinib combinations could be translated directly to the clinic, potentially improving outcomes of the patients with ovarian cancer.
Materials and Methods
Cell lines and cultures
HEY, A2780, SKOv3 and OVCAR3 cells were purchased from the American Type Culture Collection. OC316 and OVCAR8 were obtained from Dr. Gordon Mills’ laboratory. All the cell lines were validated using short tandem repeat DNA fingerprints in the MD Anderson Cancer Center Characterized Cell Line Core [supported by NIH National Cancer Institute (NCI) CCSG P30CA016672]. Mycoplasma contamination was tested periodically with a Universal Mycoplasma Detection Kit from ATCC (30-1010K). SKOv3 cells were cultured in McCoy’s 5A medium; and OVCAR3, OVCAR8 and HEY cells were grown in RPMI1640 medium. Olaparib resistant cells (IGROV1OR and OC316OR) were developed by continuous treatment of olaparib for 4 to 6 months. All cell lines were maintained in an atmosphere of 95% humidified air and 5% CO2 at 37°C.
Organoids
Organoids (2,414 and 2,445) were developed by Drs. J. Liu and X. Zhang in the Department of Anatomic Pathology, UT MD Anderson Cancer Center. Patient-derived organoids were established from xenografts derived from patients with high grade serous ovarian cancers. The UT MD Anderson Characterized Cell Line Core facility verified the source of the organoids by short tandem repeat sequence analysis.
SRB assay
Sulforhodamine B (SRB) assays were used to measure growth inhibition as previously described (10). 2,500 to 3,000 cells/well were seeded in 96-well white bottom plates and incubated overnight at 37°C in 5% CO2. Attached cells were treated for 72 to 96 hours and incubated with 0.4% SRB. Tris base was used to solubilize the SRB dye. A plate reader (BioTek Synergy Neo multi-mode) was used to measure absorbance at 510 nm. The Chou-Talalay method was used to calculate the combination Index (CI) using Compusyn software.
CellTiter-glo
2,500 to 3,000 cells/well were seeded in 96-well white bottom plates and incubated overnight at 37°C in 5% CO2. Treatment with olaparib, crizotinib or a combination of the two drugs was performed the next day. The 96-well plates were incubated at room temperature for 30 minutes prior to the addition of 100 µL of CellTiter-Glo 2.0 (Promega, G9243) per well. The plates were shaken and incubated for 10 minutes at room temperature. Luminescence was recorded using a BioTek multiplate reader.
FITC annexin-V apoptosis assay
Cells were treated with different concentrations of olaparib, crizotinib, or their combination. The percentage of apoptotic cells was measured with a FITC Annexin V Apoptosis Detection Kit I (Thermo Fisher Scientific, V13242) according to the manufacturer’s recommendations. Cell staining was analyzed on a Gallios cell analyzer (Beckman Coulter), and 20,000 events were counted.
Colony formation assays
Two hundred to 800 cells/well were seeded in six-well plates in triplicate and treated with olaparib, crizotinib or the combination of crizotinib and olaparib for 8 to 12 days. Colonies were stained and fixed with Coomassie blue (sigma, B7920), washed, and counted manually. Images were captured using a FluoChem E Imager.
Transmission electron microscopy
Cells were washed in PBS and fixed with 2.5% glutaraldehyde in 0.1 mol/L Na-cacodylate buffer and further fixed with 1% osmium tetroxide in 0.1 mol/L cacodylate buffer. Specimens were stained with aqueous uranyl acetate and lead citrate before imaging with a Jeol-100 CX II (JEOL) transmission electron microscope at 80 kV.
Measurement of intracellular ROS
A total of 1.5 to 3 × 105 cells were treated with olaparib, crizotinib or their combination and then stained with H2DCFDA (C6827; Thermo Fisher) in 1× PBS for 30 minutes at 37°C in the dark. Samples were centrifuged and re-suspended in PBS. Fluorescence intensities were then measured in a flow cytometer using the PE-Texas Red channels.
ATP assay
A2780 and OC316 cells were plated onto 12-well plate, allowed to attached overnight, and treated with olaparib and/or crizotinib for 24 hours. Total ATP levels were then measured using firefly luciferase to convert ATP and luciferin to oxyluciferin and light that could be detected (700410, Cayman Chemical).
Western blot analysis
Following different treatments over different intervals, lysates were extracted from the whole cells, and their protein concentrations were determined with BCA Kits (Bio-Rad Laboratories, Thermo Fisher Scientific, 23228). Samples with 20 µg of total protein were separated using 8% or 12% SDS-PAGE and transferred to PVDF membranes (Thermo Fisher Scientific, 88518) by electrotransfer. The membranes were then blocked with 5% BSA in TBS-Tween-20 and probed with 1:1,000 primary antibodies against LC3 (catalog 3868), ATG5 (catalog 2630), ɣ-H2AX (catalog 2577), p-AKT (catalog 9271), T-AKT (catalog 9272), p-mTOR (catalog 2971), mTOR (catalog 2972), p-ULK (catalog 6888), T-ULK (catalog 8054) and GAPDH (2118). All antibodies were purchased from Cell Signaling Technology. Antibodies were diluted in TBS-Tween-20 containing BSA and incubated at 4°C overnight. Membranes were incubated with horseradish peroxidase-conjugated 1:2,000 anti-rabbit IgG (Thermo Fisher Scientific, 31463) or 1:2,000 anti-mouse IgG secondary antibody (Thermo Fisher Scientific, 31439). Chemiluminescence was detected with ECL reagent (PerkinElmer, NEL 104001EA).
siRNA transfection
To inhibit the expression of individual genes, ON-TARGET plus pooled siRNAs (Dharmacon/Horizon) were utilized. DharmaFECT 4 reagent (T-2004-03, Dharmacon) was used to transfect with a negative control siRNA or with specific siRNAs against ATG5 or ALK. A mixture of siRNA (25 nmol/L final concentration) and DharmaFECT 4 reagent was prepared in Opti-MEM (Thermo Fisher Scientific) and incubated at room temperature for 20 minutes This mixture was added to each well and then cells were laid on top of the siRNA-DharmaFECT mixture. Cells were incubated for 48 hours and harvested to measure specific protein expression.
TUNEL staining
We employed the terminal deoxynucleotidyltransferase-mediated deoxy UTP-fluorescein nick end-labeling (TUNEL) assay using an APO-BRDU-IHC (TUNEL) Apoptosis kit (NBP2-31164) according to the manufacturer’s instruction. Briefly, following the designated treatments, tissue samples were collected and processed for paraffin embedding using standard protocols. Sections were then mounted on glass slides and stained with hematoxylin and eosin, followed by TUNEL staining. After deparaffinization and rehydration with xylene and ethanol solutions, sections were permeabilized with Proteinase K diluted in Tris pH 8.0. Subsequently, after inactivation of endogenous peroxidases, the sections were incubated with the TUNEL reaction mixture and incubated at 37°C for 1 hour. For detection, the entire specimen sections were covered with Blocking buffer for 10 minutes, followed by incubation with Biotin-Antibody solution for 1 hour and counterstaining with Methyl Green.
Immunofluorescence staining and microscopy
Cells were seeded onto sterile coverslips and treated with 5 µmol/L olaparib, 1 µmol/L crizotinib or both, for 48 hours. After treatment, cells were then fixed in 4% paraformaldehyde (PFA) for 10 minutes and permeabilized with 0.1% Triton X-100 in PBS. Cells were washed with PBS and blocked using 5% BSA for 45 minutes. Samples were then stained overnight at 4°C with rabbit anti-phospho-histone H2AX (Ser139) monoclonal antibody (1:1,000) or LC3B (1:400) antibody. After washing, cells were incubated with Alexa Fluor 488-labeled goat anti-rabbit antibody for 1 hour at room temperature. Nuclei were stained with DAPI for 10 minutes Coverslips were mounted with Fluoro-Gel with TES (Electron Microscopy Science, 50-246-96) and air-dried. Transmission electron microscopy (TEM) images were obtained with an Olympus IX71 microscope and the Olympus CellSens Dimension software.
IHC staining
We performed immunohistochemistry on formalin-fixed paraffin-embedded (FFPE) mouse tissue sections. The sections underwent deparaffinization and rehydration using ethanol solutions. Antigens were retrieved using Rodent Decloaker (BioCare Medical, RD913M) and microwave treatment. Sequential blocking steps involved PeroxAbolish and Rodent Block M. Primary antibodies targeting LC3 and ɣ-H2AXwere incubated overnight at 4°C. Detection was achieved using VisUCyte HRP Polymer IgG (VC003-025), followed by incubation with DAB substrate. Counterstaining was performed with CAT hematoxylin, and the slides were sealed with Permount after dehydration.
Human ovarian cancer cell line xenografts
All animal experiments with female athymic nu/nu mice were conducted following protocols approved by the Institutional Animal Care and Use Committee (IACUC ID: 00001195) at the M. D. Anderson Cancer Center. OVCAR8 and OC316 cells were implanted intraperitoneally or subcutaneously in female nu/nu mice aged 6 to 8 weeks. Mice were randomized into four treatment groups: (i) vehicle control (4% DMSO with 30% polyethylene glycol (PEG) 300 and double distilled H2O, (ii) olaparib (40 mg/kg 5 days/week ip), (iii) crizotinib (20 mg/kg 5 days/week by oral gavage) and (iv) olaparib and crizotinib (5 days/week) for a duration of 4 to 6 weeks. At the end of the experiment. tumor weight was measured to assess treatment efficacy. Throughout the study, the well-being of the mice was closely monitored for signs of dyspnea, weight loss, hunched posture, snuffling respiratory sounds or abdominal breathing were detected. Upon reaching ethical endpoint, euthanasia was performed.
Statistical Analysis
We used GraphPad prism software for statistical analysis. All cellular assays were repeated independently 2 or 3 times. Data are presented as the mean and standard deviation (SD) or standard error (SE). Significance of differences was assessed using ANOVA. The criterion for statistical significance was taken as P less than 0.05. For the OVCAR8 and OC316 Xenograft models we used One-way ANOVA or Two-way ANOVA for comparison of the different groups.
Data availability
The data generated in this study are available upon request from the corresponding author.
Results
Combined treatment with olaparib and crizotinib synergistically inhibits ovarian cancer cell growth
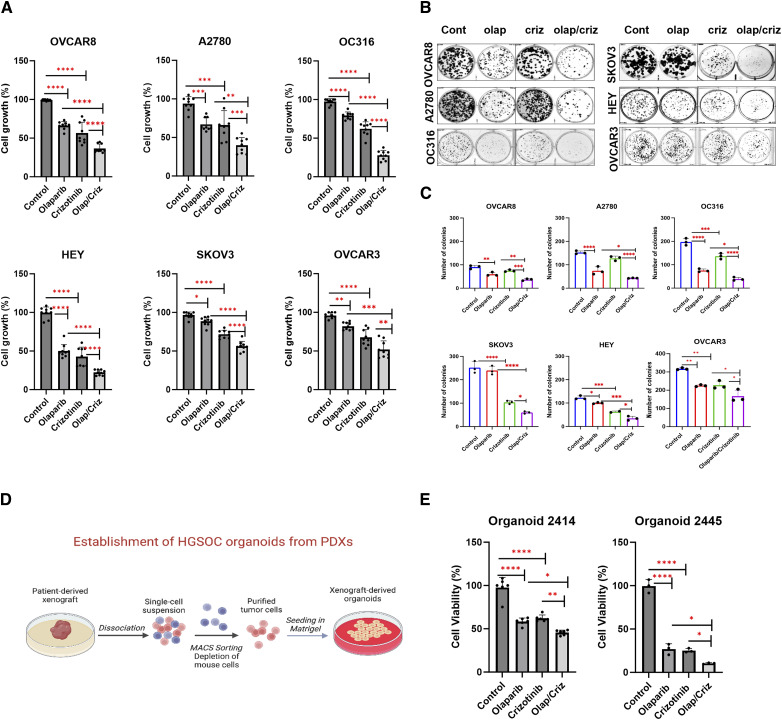
To test whether treatment with crizotinib could increase the efficacy of olaparib, we examined the effect of combining crizotinib with different PARPi on cell growth in six ovarian cancer cell lines (OVCAR8, A2780, OC316, SKOv3, HEY and OVCAR3) and two olaparib resistant cancer cell lines (IGROV1R and OC316R), as well as two patient-derived ovarian cancer organoids (2,414 and 2,445). Crizotinib and olaparib in combination exhibited synergistic growth inhibition [combination index (CI) <1] in all six cell lines, with greater growth inhibition by the combination than with either single agent alone (Fig. 1A; Supplementary Fig. S1A). This synergistic effect was confirmed by a significant shift of IC50 to a lower value in, HEY, OC316 and OVCAR8 compared to olaparib alone (Supplementary Fig. S1B). Additionally, the crizotinib/olaparib combination produced greater inhibition of colony formation in clonogenic assays compared with either single agent (Fig. 1B and C). We further confirmed the inhibitory effect of crizotinib/olaparib on tumor cell growth in patient-derived ovarian cancer organoids (Fig. 1D and E) and found that crizotinib overcame olaparib acquired resistance in two olaparib-resistant ovarian cancer cell lines that were established by continuous treatment of olaparib for 4 to 6 months (Supplementary Fig. S2). Moreover, this synergistic effect was observed not only with olaparib, but also with other PARPi, including niraparib, rucaparib and talazoparib. (Supplementary Fig. S3). Altogether, our findings suggest that combining crizotinib with PARPi could provide a promising strategy for the treatment of ovarian cancer.
Figure 1.
Crizotinib enhances olaparib sensitivity in ovarian cancer cells and ovarian patient-derived organoids. A, The effect of olaparib and crizotinib was evaluated by cell viability assays. Ovarian cancer cells were treated with 5 µmol/L olaparib (olap), 1 µmol/L of crizotinib (criz) or both for 5 days, followed by fixation and staining using sulforhodamine B (SRB). Data represent results from three independent experiments with three replicates. (B and C) Growth inhibition was evaluated using colony formation assays. Cells were seeded and cultured with olaparib, crizotinib, or olaparib + crizotinib in six well plates for 8–12 days and stained with Coomassie (blue; B). Quantification of colonies (>50 cells) was presented in six ovarian cancer cell lines (C). Data represent results from one independent experiment with three replicates. Experiments were repeated with similar results. D, The process of establishing organoids from patient-derived xenografts (PDXs) is illustrated. The image was created with Biorender. Adapted from Zhang and colleagues (20). E, Patient-derived organoid 2,414 was treated with olaparib (20 µmol/L) and crizotinib (1.5 µmol/L) and organoid 2,445 was treated with olaparib (0.5 µmol/L) and crizotinib (1 µmol/L). CellTiter-glo was used to measure cell viability. Data represents the means of two independent experiments for organoid 2,414 and one independent experiment for organoid 2,445 with three replicates. Results were analyzed using GraphPad Prism. The statistical significance was determined with one-way ANOVA. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. Error bars represent means ± SD. (D, Created with BioRender.com.)
Combined treatment with olaparib and crizotinib induces autophagy in ovarian cancer cells
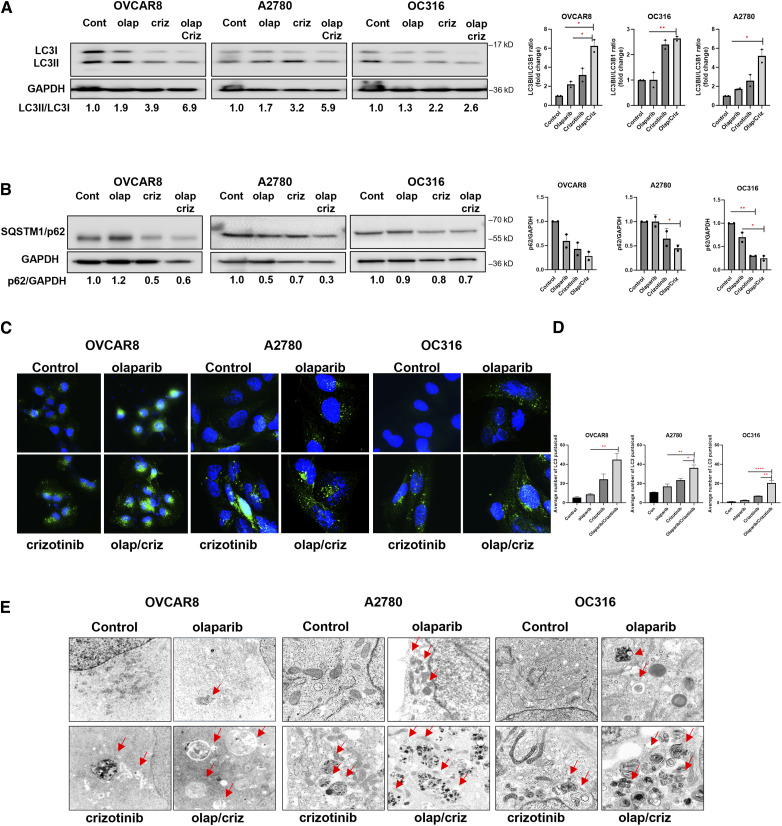
Previous studies from our laboratory have demonstrated that olaparib and crizotinib can individually induce autophagy in ovarian cancer cells (5, 6). During induction of autophagy, microtubule associated protein chain 3 (LC3-I), a ubiquitin-like molecule, is cleaved by ATG4 protease and subsequently conjugated with phosphatidylethanolamine (PE) producing LC3-II, which is translocated from the cytoplasm to the autophagosome membrane. Based on the importance of LC3 processing for autophagosome formation and function, the conversion of LC3-I to LC3-II is widely used as a marker of autophagy induction (11). To determine the combined effects of olaparib and crizotinib on the induction of autophagy, we measured LC3I to LC3II conversion in six ovarian cancer cell lines (Fig. 2A–D; Supplementary Fig. S4A). Treatment with olaparib and crizotinib in combination increased conversion of LC3I to LC3II, indicating induction of autophagy. Furthermore, we evaluated autophagy flux by measuring decreases in p62 expression. A greater reduction in p62 expression was produced by treatment with a combination of olaparib and crizotinib than with either individual drug providing additional evidence for the induction of autophagic flux (Fig. 2B; Supplementary Fig. S4B).
Figure 2.
Combination of Olaparib and crizotinib induces autophagy in ovarian cancer cells. A and B, Analysis of LC3 and p62 protein level. Cells were treated with olaparib (olap, 5 µmol/L) and/or crizotinib (criz, 1 µmol/L). Cell lysates were collected, and Western blots were performed to examine the conversion of LC3-I to LC3-II and the p62 expression. The numbers below the gel lanes represent the relative protein levels determined from band intensities. Ratios of LC3-II/LC3-I and SQSTM1/p62 to GAPDH were calculated using ImageJ and normalized to the control. C, Analysis of autophagy by immunofluorescence staining of punctate LC3. Cells were plated in chamber slides and treated with olaparib (5 µmol/L) and crizotinib (1 µmol/L) for 48 hours. Representative images are presented here. Scale bar: 20 µm. Green dots indicate LC3 and (blue; DAPI) indicate nuclear staining. D, The average of LC3 puncta per cell was calculated by analyzing >100 cells per condition using ImageJ. Statistical analysis was conducted using one-way ANOVA. Error bars represent mean ± SD. E, Measurement of olaparib/crizotinib-induced autophagy with transmission electron microscopy (TEM) in OVCAR8, A2780 and OC316 cells. Red arrows indicate typical membrane autophagosomes.
Immunofluorescence staining of microtubule-associated protein 1 light chain 3 beta (MAP-LC3B) was used to visualize autophagosomes. Combined treatment with olaparib and crizotinib increased the number of ovarian cancer cells with green, fluorescent LC3 punctae compared to diluent-treated control groups, indicating the presence of autophagosomes and the accumulation of LC3 in autophagic vesicles (Fig. 2C and D; Supplementary Fig. S4C). Finally, we observed double membrane autophagic vesicles in all six ovarian cancer cell lines via transmission electron microscopy (TEM). The number of autophagic vacuoles was greater in cells treated with both olaparib and crizotinib than in control groups (Fig. 2E; Supplementary Fig. S4D), providing further evidence that the olaparib/crizotinib combination induces autophagy in ovarian cancer cells.
Olaparib/crizotinib treatment induces autophagy-associated cell death
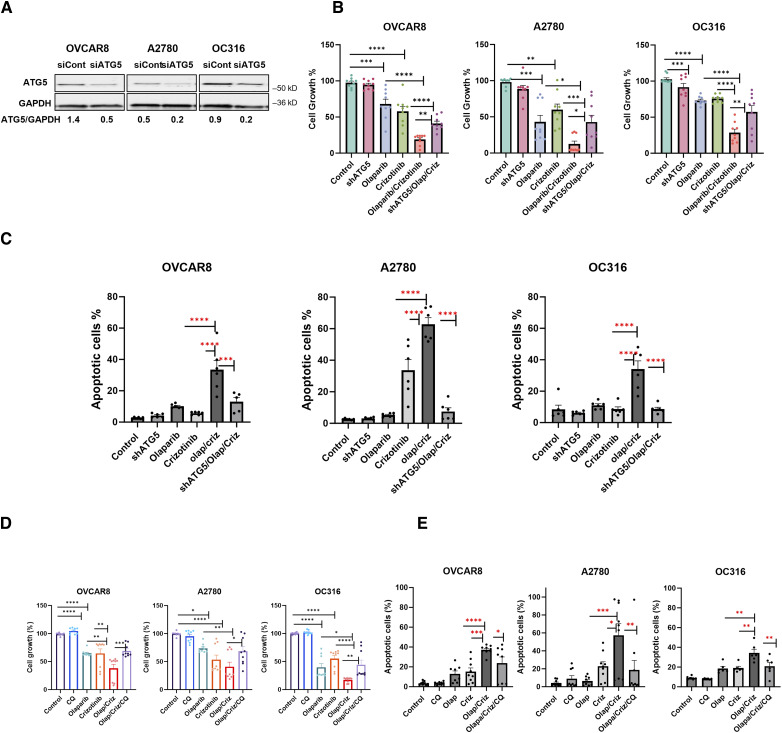
Since olaparib induces autophagy and treatment with crizotinib in olaparib-treated cells further increases autophagy (Fig. 2A–D; Supplementary Fig. S4), we explored whether excessive autophagy plays a role in crizotinib/olaparib-induced cytotoxicity/apoptosis. Knockdown of ATG5, a critical protein required for the formation of autophagosomes, decreased sensitivity of OVCAR8, OC316 and A2780 cells to treatment with olaparib and crizotinib in combination as shown by an increase in cell viability (Fig. 3A and B) and a decrease in olaparib/crizotinib-induced apoptosis (Fig. 3C). Moreover, the addition of the pharmacologic inhibitor of autophagy chloroquine (CQ) to olaparib/crizotinib treatment significantly decreased the sensitivity of ovarian cancer cell lines, leading to an increase in cell growth and a decrease in apoptosis (Fig. 3D and E). Altogether, these data suggest that excessive autophagy contributes, at least in part, to the cytotoxicity and apoptosis induced by crizotinib/olaparib treatment.
Figure 3.
Inhibition of olaparib/crizotinib-induced autophagy results in increased cell growth and decreased apoptosis. A and B, Assessment of the effect of autophagy inhibition in ovarian cancer cells. OVCAR8, A2780 and OC316 were transfected with siControl or ATG5 siRNA (20 nmol/L). Knockdown efficacy was observed by Western blot analysis. Numbers below the gel lanes represent the relative protein level, determined from the band intensity (A). Cell viability was examined in OVCAR8, A2780 and OC316 cell line. 3,000 cells per well were plated onto 96-well plates, then sequentially transfected with ATG5 siRNA (20 nmol/L). Olaparib and/or crizotinib were added 24 hours post-transfection daily for 5 days. Cells were then fixed and stained by sulforhodamine B (SRB). Representative data are from three experiments with three replicates (B). C, Cells were labeled with PI/annexin V-FITC and analyzed for apoptosis by flow cytometry. Representative data is from two experiments with three replicates. D, Three thousand cells per well were plated onto 96-well plates and were then treated with CQ (3 µmol/L) for 30 mins with or without olaparib, crizotinib or both for 5 days. After incubation, cells were fixed and stained by sulforhodamine B (SRB). Representative data is from three experiments with three replicates. E, Detection of apoptosis was performed by using Annexin/propidium iodide (PI) staining. Cells were treated with olaparib (5 µmol/L), olaparib (5 µmol/L) alone or combined for 5 days. Representative data are from three experiments with three replicates for OVCAR8 and A2780 and two experiments with three replicates for OC316. Results were obtained using one-way ANOVA. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. Error bars represent means ± SEM.
Crizotinib enhances olaparib-induced ROS, DNA damage and apoptosis
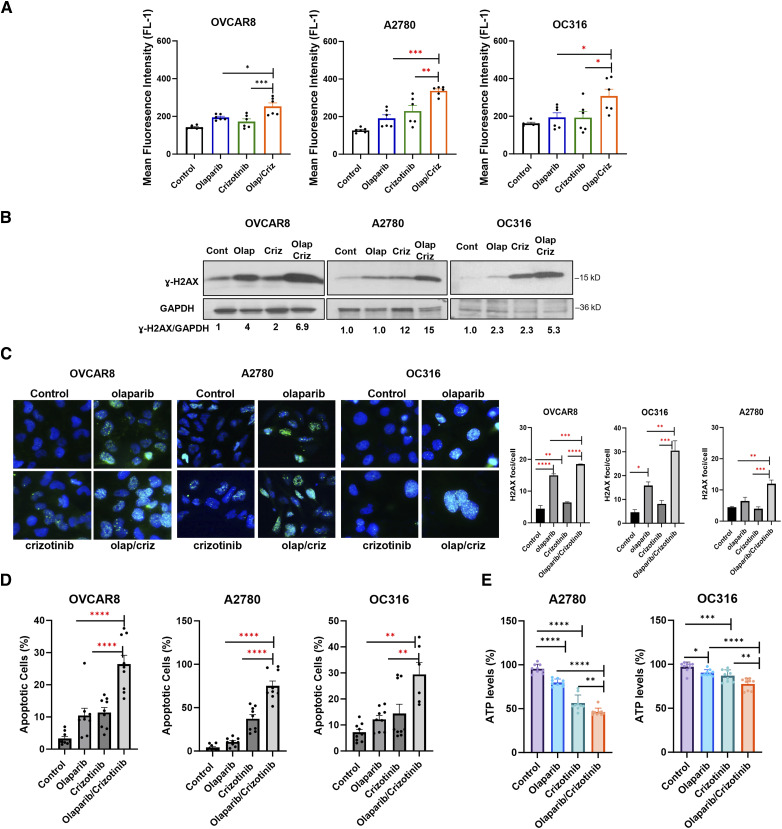
As previous studies indicated that both crizotinib and olaparib enhance reactive oxygen species (ROS)-induced cell death (5, 12), we hypothesized that crizotinib may increase olaparib’s growth inhibitory function by enhancing olaparib induced ROS production in ovarian cancer. To test this possibility, we examined ROS production in ovarian cancer cells treated with crizotinib, olaparib or the combination and found that crizotinib significantly increased olaparib-induced ROS, judged by increasing CM-H2DCFDA-positive cells treated with olaparib/crizotinib compared to cells treated only with olaparib (Fig. 4A; Supplementary Fig. S5A). With high ROS inducing DNA damage and apoptosis, we also measured ɣ-H2AX, a marker of DNA damage and apoptosis in cells treated with crizotinib, olaparib and the crizotinib/olaparib combination. We showed that crizotinib/olaparib induced greater DNA damage (Fig. 4B and C; Supplementary Fig. S5B and S5C) and apoptosis (Fig. 4D; Supplementary Fig. S5D) than olaparib alone, judged by increased ɣ-H2AX and Annexin V, respectively. In addition, as ATP production could be affected by ROS levels, we also measured ATP and found that olaparib/crizotinib treatment decreased ATP levels compared with control, olaparib treatment alone or crizotinib treatment alone (Fig. 4E). Taken together, these results suggest that crizotinib synergizes with olaparib by promoting ROS production, inducing DNA damage, and decreasing ATP levels, ultimately leading to apoptosis.
Figure 4.
Combination of olaparib and crizotinib induces ROS, DNA damage and apoptosis in ovarian cancer cells. A, ROS formation was evaluated by plating cells treated with olaparib (5 µmol/L) and crizotinib (1 µmol/L) for 48 hours in triplicate in six-well paltes. Cells were then stained with 5 mmol/L H2DCFDA, collected, and analyzed by flow cytometry after a 30-minute incubation. ROS formation is represented as Mean fluorescence intensity. The data represent the mean of two independent experiments with three replicates. Statistical analysis was conducted using one-way ANOVA. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Error bars represent mean ± SEM. B and C, Evaluation of ɣ-H2AX by Western blot analysis (B) and fluorescence staining (green dots; C). Cancer cells were treated with olaparib (5 µmol/L), crizotinib (1 µmol/L) of both for 5 days. D, Cells were labeled with PI/Annexin V-FITC and analyzed for apoptosis using flow cytometry. Error bars represent means ± SEM. E, Measurement of ATP levels in A2780 and OC316 cells. Cells were treated with olaparib and/or crizotinib, and ATP levels were measured after 24 hours treatment, using ATP Detection Assay-Luminescence from Cayman Chemical. Data include means of three independent experiments with three replicates. Results were obtained using one-way ANOVA. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. Error bars represent mean ± SD.
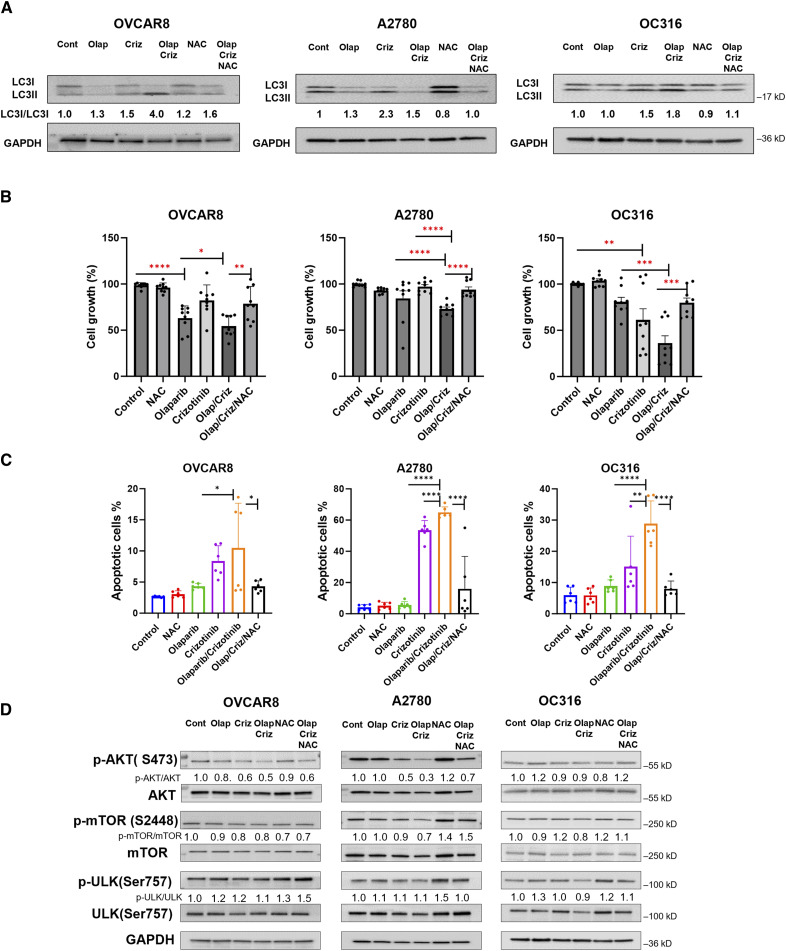
Olaparib/crizotinib induced autophagy-associated cell death through ROS
Our previous studies have documented that olaparib induces autophagy, partly through ROS generation (5). We asked whether ROS plays a role in olaparib/crizotinib induced autophagy, using N-Acetylcysteine (NAC), a scavenger of ROS. Western blot analysis revealed that the conversion of LC3I/LC3II induced by the combination of olaparib/crizotinib was reduced in cells treated with NAC (Fig. 5A). We then examined the contribution of ROS to olaparib/crizotinib-induced cell death. Cell viability assays and flow cytometry analysis demonstrated that the co-treatment with NAC attenuated the decrease in cell growth and reversed olaparib/crizotinib-induced apoptosis (Fig. 5B and C). Thus, these results suggest that in addition to inducing DNA damage, ROS may contribute to autophagy induction and cell death in the tumor cell treated with olaparib/crizotinib.
Figure 5.
Inhibition of olaparib/crizotinib-induced ROS increases cell growth, decreases apoptosis and inhibits autophagy. A, Assessment of the effect of ROS inhibition on induction of autophagy. OVCAR8, A2780 and OC316 cells were treated with olaparib/crizotinib for a total of 48 hours and NAC (1 mmol/L) was added to culture media 24 hours before analysis. Western blot analysis was performed to evaluate conversion of LC3I/LC3II. B, Measurement of cell viability with and without NAC treatment. Cells were treated with olaparib, crizotinib and/or NAC for 5 days. After incubation, cells were fixed and stained by sulforhodamine B (SRB). C, Analysis of apoptosis with and without NAC treatment. Cells were labeled with PI/annexin V-FITC and analyzed for apoptosis by flow cytometry. Data represents results from two independent experiments with three replicates. Results were obtained using one-way ANOVA. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. D, Evaluation of the expression of AKT/mTOR-ULK1 after treatment with olaparib, crizotinib, NAC or combination for 48 hours, NAC was added 24 hours before analysis. Numbers below the gel lanes represent the relative protein level, determined from the band intensity. GAPDH for (D) from OC316 cells is the same as that presented for the same cells in (A) since the blots in each panel were performed using the same membranes.
The AKT/mTOR/ULK1 pathway is involved in ROS mediated autophagic cell death
Studies have suggested AKT and mTOR are regulated by ROS and that ROS can suppress AKT and mTOR (13, 14). Therefore, we investigated the involvement of the AKT/mTOR pathway in ROS-mediated autophagic cell death following treatment with olaparib and crizotinib. Western blot results revealed that olaparib/crizotinib treatment decreased phosphorylation of AKT, mTOR and ULK (Ser757), while the ROS scavenger NAC rescued the olaparib/crizotinib-induced decrease in p-AKT, p-mTOR and p-ULK-1 levels (Fig. 5D). Taken together, these results suggest that a combination of olaparib and crizotinib reduces AKT signaling, inducing autophagy, and increasing apoptosis in ovarian cancer cells. Also, these data indicate that the olaparib/crizotinib-mediated suppression of the AKT-mTOR-ULK1 pathway is dependent on ROS generation and is involved in the autophagy-associated cell death induced by olaparib/crizotinib.
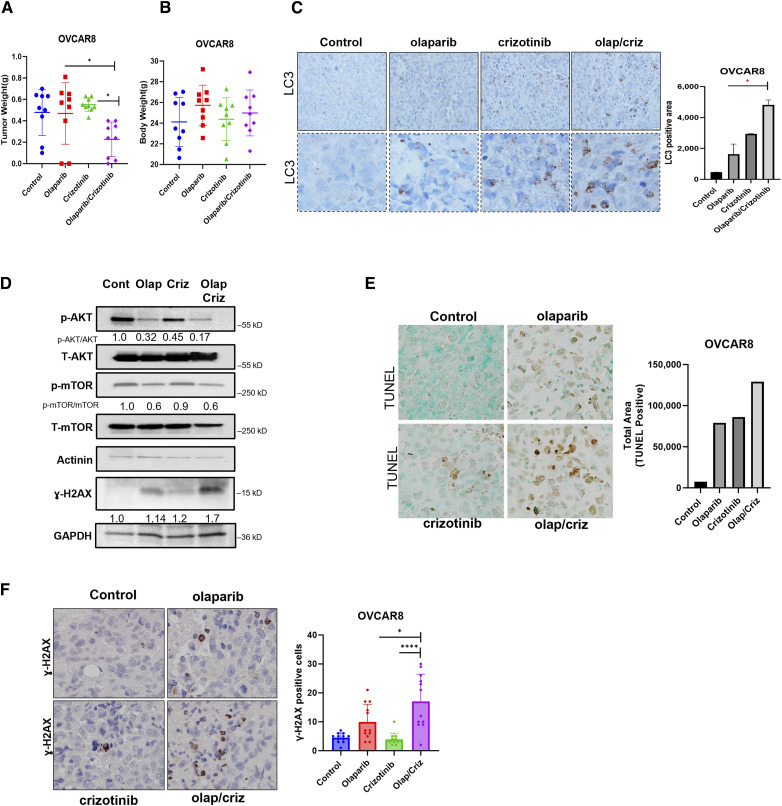
Crizotinib enhances the cytotoxic effect of olaparib in ovarian cancer cell line-derived human ovarian cancer xenografts
Given the enhanced cytotoxicity that was observed in cultured cells treated with olaparib and crizotinib in combination, we tested whether crizotinib could augment the response to treatment with olaparib in two xenograft models. To reflect the original microenvironment, OVCAR8 cells were injected intraperitoneally into nude mice. Mice were randomized into four treatment groups as indicated in Fig. 6B. We found that the combination of crizotinib and olaparib significantly decreased tumor growth compared to monotherapy groups of olaparib and crizotinib, (Fig. 6A). Importantly, no significant changes in body weight were observed among any of the experimental groups throughout the 6 weeks of treatment, as compared to the control group (Fig. 6B). When the OVCAR8 cell line was injected subcutaneously into mice, treatment with olaparib and crizotinib, significantly inhibited tumor growth compared to either single agent alone (Supplementary Fig. S6A). Similar results were obtained in the OC316 human ovarian cancer cell line (Supplementary Fig. S6B), with no observable changes in body weight (Supplementary Fig. S6C). To confirm the mechanisms by which crizotinib/olaparib inhibited tumor cell growth and enhanced cell death in vivo, tumor tissues were collected for histological analysis by immunohistochemistry (IHC), Results from the OVCAR8 xenograft studies recapitulated in vitro studies. A significant accumulation of punctate LC3 was observed after treatment with a combination of olaparib and crizotinib (Fig. 6C), indicating induction of autophagy. Additionally, a decrease in p-AKT and p-mTOR was observed, consistent with the induction of autophagy (Fig. 6D). TUNEL staining revealed a higher percentage of TUNEL-positive cells in xenografts treated with the combination of the both drugs compared to the individual drug-treated groups (Fig. 6E). Consistent with these findings, an increase in ɣ-H2AX was also observed with the combination treatment, indicating induction of DNA damage. (Fig. 6D and F). These results suggest that crizotinib sensitizes ovarian cancer xenografts to the cytotoxic effects of olaparib and the combination treatment induces both autophagy and apoptosis, thereby inhibiting tumor cell growth and inducing cell death in vivo. Collectively, these preclinical models underscore the potential of the combination of olaparib with crizotinib as a novel therapeutic strategy that could benefit ovarian cancer patients.
Figure 6.
Combination of Olaparib + crizotinib decreases tumor growth, induces autophagy and apoptosis in OVCAR8 xenograft model. A and B, Evaluation of the combination of olaparib/crizotinib in ovarian cancer xenograft models of OVCAR8. OVCAR8 cells (2.5 × 106) were injected intraperitoneally into nu/nu mice that were treated daily with 40 mg/kg olaparib ip, 15 mg/kg crizotinib oral gavage or both for 6 weeks. At the end of the experiment, mice were sacrificed, and tumor weight (A) and body weight (B) were measured. C, Detection of punctate LC3-staining by immunohistochemistry using paraffin-embedded sections of tumor excised from mice from each group. Pictures with dashed borders are zoomed and cropped sections. LC3 positive area from different random fields with at least 100 cells from every group were counted. D, Western blot analysis of autophagy and apoptosis markers as indicated. Quantification of protein signals were performed with ImageJ. E, Induction of apoptosis in vivo was evaluated using TUNEL staining. Brown staining indicates TUNEL-positive nuclei and blue staining indicates TUNEL-negative nuclei. Total area of positive TUNEL staining of at least 100 cells was calculated with ImageJ. F, Representative images of IHC for ɣ-H2AX from different mouse tumor tissue sections with more than 100 cells. Analysis was determined by one-way ANOVA. **, P < 0.01; ***, P < 0.001. Error bars represent means ± SD.
Discussion
In recent years, PARP inhibitors have improved disease free and overall survival in a fraction of women with ovarian cancer. The development of resistance to PARPi is, however, a major obstacle to their clinical efficacy (15). In this study, we investigated the potential of crizotinib to enhance the therapeutic efficacy of different PARP inhibitors in a panel of ovarian cancer cell lines, patient derived organoids and olaparib resistant ovarian cancer cells. Overall, our findings demonstrate that the combination of crizotinib with olaparib and other PARP inhibitors - including rucaparib, niraparib and talazoparib - results in synergistic cytotoxicity against ovarian cancer cell lines in culture. Moreover, this combination showed superior efficacy compared to either single agent in two human xenograft models of the OVCAR8 and OC316 human ovarian cancer cell lines.
Resistance to PARPi can arise from several different mechanisms, including induction of autophagy (5). Autophagy is a process that responds to stressful conditions, such as nutrient deprivation, hypoxia, or chemotherapy. Our study revealed that treatment with crizotinib can increase autophagy induced by olaparib in several ovarian cancer cell lines and xenograft models. These results suggest that the combination of crizotinib and PARPi may be an effective strategy for treating PARPi-resistant ovarian cancer by inducing autophagy-associated cell death.
Interestingly, autophagy can play both tumor-suppressive and promoting roles depending on the cellular context (8, 16). In a previous study, we documented for the first time that PARPi (olaparib, rucaparib, niraparib and talazoparib) can induce autophagy in different models of ovarian cancer, which provides an adaptive mechanism of PARPi resistance (5). In accordance with this, Cahuzac and colleagues, also demonstrated that autophagy can contribute to the sensitivity of pancreatic cancer cells to olaparib by regulating p62 nuclear localization (17), while Ozpolat and colleagues, showed that olaparib induces autophagy/mitophagy in breast cancer cells and it is involved in induction of cell death (18).
As olaparib induces autophagy and treatment with crizotinib in olaparib-treated cells further increases autophagy, we investigated whether excessive autophagy after treatment with crizotinib and olaparib results in autophagy-associated cell death. We demonstrated that treatment with siRNA against ATG5 in OVCAR8, OC316 and A2780 cells increased cell viability and decreased apoptosis after treatment with olaparib/crizotinib, suggesting that autophagy-associated cell death was implicated. These results were confirmed by pharmacologic inhibition of autophagy using chloroquine.
Regulation of autophagy is complex, and accumulating evidence suggests that oxidative stress could be a factor in inducing both apoptosis and autophagy (19). Consistent with our previous studies (5), we found that olaparib induces formation of ROS. Moreover, the combination of olaparib/crizotinib further increased ROS levels and decreased ATP levels. In line with these observations, our results also showed an increase in apoptosis and in ɣ-H2AX expression and focus formation upon combination treatment, indicating increased DNA damage.
The PI3K/AKT/mTOR pathway is a crucial negative regulator of autophagy. In our study, AKT phosphorylation decreased. This decrease in AKT phosphorylation was associated with a decrease in p-mTOR and p-ULK(Ser757), both of which contribute to an increase in autophagy after olaparib/crizotinib treatment, consistent with the possibility that olaparib/crizotinib-induced autophagy is mediated by inhibiting the AKT/mTOR/ULK1 pathway. Previous studies have shown that ROS can directly dephosphorylate AKT, resulting in its inactivation (13). However, the relationship between ROS, AKT and autophagy following olaparib/crizotinib treatment has not previously been studied. Our data suggest that ROS production was involved in the olaparib and crizotinib-induced autophagy via inactivation of the AKT/mTOR/ULK1 signaling pathway, and that olaparib and crizotinib-induced ROS plays an important role in autophagy induction. Interestingly, inhibition of ROS with NAC reversed the olaparib and crizotinib-induced decrease in p-AKT, p-mTOR and p-ULK (Ser757) and inhibited autophagy indicated by decreased in LC3 conversion. Our data suggest that ROS is an upstream regulator of the AKT pathway, and that the combination of olaparib and crizotinib synergistically induces autophagy-mediated cell death and apoptosis of ovarian cancer cells through the generation of ROS and inactivation of the AKT/mTOR/ULK1 signaling pathway. This, inhibition of ROS significantly decreased the antiproliferative effects of olaparib/crizotinib treatment by increasing cell growth and decreasing apoptosis after treatment with the combination.
To elucidate the mechanism underlying the synergistic impact of olaparib and crizotinib, we utilized ALK siRNA to determine whether crizotinib’s effect is mediated by ALK inhibition. The depletion of ALK enhanced the sensitivity of ovarian cancer cells to olaparib. Moreover, the knockdown of ALK further augmented the combination of olaparib/crizotinib, suggesting that crizotinib’s effect might extend beyond ALK inhibition to other targets (Supplementary Fig. S7). However, this study did not evaluate other kinases such as ROS1 or c-MET, which are known targets of crizotinib. In summary, our study demonstrates that the combination of olaparib and crizotinib induced ROS formation and enhanced olaparib-induced autophagy, leading to cell death and tumor regression. These findings provide a potential therapeutic strategy for improving the efficacy of PARPi and the overall outcome for ovarian cancer patients.
Supplementary Material
Supplementary Figure S1 shows CI values and IC50 of Olaparib and Olaparib/crizotinib
Supplementary Figure S2 shows dose response curves, colony formation assays and viability of Olaparib resistant cells treated with Olaparib and crizotinib
Supplementary Figure S3 shows the viability of ovarian cancer cells treated with other PARPi in combination with Olaparib
Supplementary Figure S4 shows induction of autophagy after treatment with the combination of Olaparib and crizotinib
Supplementary Figure S5 shows induction of ROS, DNA damage and apoptosis after treatment with Olaparib and crizotinib
Supplementary Figure S6 shows the effect of the combination of Olaparib and crizotinib in tumor growth
Supplementary Figure S7 shows the effect of knocking down ALK on the sensitivity of ovarian cancer cells to Olaparib/crizotinib combination
Acknowledgments
The authors would like to thank Mr. Kenneth Dunner Jr at the UT MD Anderson High Resolution Electron Microscopy Facility for assistance with electron microscopy. We also thank Karen Ramirez, David Dwyer, and Sarah Schneider for providing assistance with flow cytometry. R01 CA 135354 (R.C. Bast PI), R01CA266187 (R.C. Bast PI), NIH/NCI Diversity Supplement #R01CA135354 (J.M. Santiago-O’Farrill), the MD Anderson SPORE in Ovarian Cancer NCI P50 CA 83639 (Bast and Sood MPI), the Shared Resources of the MD Anderson CCSG grant NCI P30 CA16672 (P Pisters PI), generous philanthropic support from Stuart and Gaye-Lynn Zarrow, the Zarrow Foundation, the Mossy Foundation and the Roberson Endowment, the Ovarian Cancer Research Alliance (OCRA) and Joseph and Angela Campolo.
Footnotes
Note: Supplementary data for this article are available at Molecular Cancer Research Online (http://mcr.aacrjournals.org/).
Authors’ Disclosures
A. Blessing Bollu reports grants from MDACC Odyssey Fellowship supported by the CFP Foundation during the conduct of the study; and is a current employee and shareholder of Johnson and Johnson Innovative Medicine and a former employee of Clovis Oncology. No disclosures were reported by the other authors.
Authors’ Contributions
J.M. Santiago-O’Farrill: Conceptualization, data curation, formal analysis, supervision, validation, investigation, visualization, methodology, writing–original draft, writing–review and editing. A. Blessing Bollu: Conceptualization, data curation, formal analysis, supervision, validation, investigation, visualization, methodology, writing–review and editing. H. Yang: Validation, investigation, visualization, methodology. V. Orellana: Data curation, investigation. M. Pina: formal analysis, investigation. X. Zhang: Methodology. J. Liu: Investigation, methodology. R.C. Bast: Conceptualization, resources, data curation, formal analysis, supervision, funding acquisition, validation, investigation, visualization, methodology, writing–original draft, project administration, writing–review and editing. Z. Lu: Conceptualization, resources, data curation, formal analysis, supervision, funding acquisition, validation, investigation, visualization, methodology, writing–original draft, project administration, writing–review and editing.
References
- 1. Bowtell DD, Bohm S, Ahmed AA, Aspuria PJ, Bast RC Jr, Beral V, et al. Rethinking ovarian cancer II: reducing mortality from high-grade serous ovarian cancer. Nat Rev Cancer 2015;15:668–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Loizzi V, Ranieri G, Laforgia M, Gadaleta CD, Gargano G, Kardhashi A, et al. PARP inhibitors and epithelial ovarian cancer: molecular mechanisms, clinical development and future prospective. Oncol Lett 2020;20:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ray Chaudhuri A, Nussenzweig A. The multifaceted roles of PARP1 in DNA repair and chromatin remodelling. Nat Rev Mol Cell Biol 2017;18:610–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pilie PG, Gay CM, Byers LA, O’Connor MJ, Yap TA. PARP inhibitors: extending benefit beyond BRCA-mutant cancers. Clin Cancer Res 2019;25:3759–71. [DOI] [PubMed] [Google Scholar]
- 5. Santiago-O’Farrill JM, Weroha SJ, Hou X, Oberg AL, Heinzen EP, Maurer MJ, et al. Poly(adenosine diphosphate ribose) polymerase inhibitors induce autophagy-mediated drug resistance in ovarian cancer cells, xenografts, and patient-derived xenograft models. Cancer 2020;126:894–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blessing AM, Santiago-O’Farrill JM, Mao W, Pang L, Ning J, Pak D, et al. Elimination of dormant, autophagic ovarian cancer cells and xenografts through enhanced sensitivity to anaplastic lymphoma kinase inhibition. Cancer 2020;126:3579–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chun Y, Kim J. Autophagy: an essential degradation program for cellular homeostasis and life. Cells 2018;7:278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Santiago-O’Farrill JM, Kleinerman ES, Hollomon MG, Livingston A, Wang WL, Tsai JW, et al. Phosphorylated heat shock protein 27 as a potential biomarker to predict the role of chemotherapy-induced autophagy in osteosarcoma response to therapy. Oncotarget 2018;9:1602–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eskelinen EL. The dual role of autophagy in cancer. Curr Opin Pharmacol 2011;11:294–300. [DOI] [PubMed] [Google Scholar]
- 10. Vichai V, Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc 2006;1:1112–6. [DOI] [PubMed] [Google Scholar]
- 11. Yoshii SR, Mizushima N. Monitoring and measuring autophagy. Int J Mol Sci 2017;18:1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kanakkanthara A, Hou X, Ekstrom TL, Zanfagnin V, Huehls AM, Kelly RL, et al. Repurposing ceritinib induces DNA damage and enhances PARP inhibitor responses in high-grade serous ovarian carcinoma. Cancer Res 2022;82:307–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wen C, Wang H, Wu X, He L, Zhou Q, Wang F, et al. ROS-mediated inactivation of the PI3K/AKT pathway is involved in the antigastric cancer effects of thioredoxin reductase-1 inhibitor chaetocin. Cell Death Dis 2019;10:809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li M, Zhao L, Liu J, Liu A, Jia C, Ma D, et al. Multi-mechanisms are involved in reactive oxygen species regulation of mTORC1 signaling. Cell Signal 2010;22:1469–76. [DOI] [PubMed] [Google Scholar]
- 15. Dias MP, Moser SC, Ganesan S, Jonkers J. Understanding and overcoming resistance to PARP inhibitors in cancer therapy. Nat Rev Clin Oncol 2021;18:773–91. [DOI] [PubMed] [Google Scholar]
- 16. Yun CW, Jeon J, Go G, Lee JH, Lee SH. The dual role of autophagy in cancer development and a therapeutic strategy for cancer by targeting autophagy. Int J Mol Sci 2020;22:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cahuzac M, Langlois P, Peant B, Fleury H, Mes-Masson AM, Saad F. Pre-activation of autophagy impacts response to olaparib in prostate cancer cells. Commun Biol 2022;5:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Arun B, Akar U, Gutierrez-Barrera AM, Hortobagyi GN, Ozpolat B. The PARP inhibitor AZD2281 (olaparib) induces autophagy/mitophagy in BRCA1 and BRCA2 mutant breast cancer cells. Int J Oncol 2015;47:262–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen Y, McMillan-Ward E, Kong J, Israels SJ, Gibson SB. Oxidative stress induces autophagic cell death independent of apoptosis in transformed and cancer cells. Cell Death Differ 2008;15:171–82. [DOI] [PubMed] [Google Scholar]
- 20. Zhang X, Yao J, Li X, Niu N, Liu X, Hajek RA, et al. Targeting polyploid giant cancer cells potentiates a therapeutic response and overcomes resistance to PARP inhibitors in ovarian cancer. Sci Adv 2023;9:eadf7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1 shows CI values and IC50 of Olaparib and Olaparib/crizotinib
Supplementary Figure S2 shows dose response curves, colony formation assays and viability of Olaparib resistant cells treated with Olaparib and crizotinib
Supplementary Figure S3 shows the viability of ovarian cancer cells treated with other PARPi in combination with Olaparib
Supplementary Figure S4 shows induction of autophagy after treatment with the combination of Olaparib and crizotinib
Supplementary Figure S5 shows induction of ROS, DNA damage and apoptosis after treatment with Olaparib and crizotinib
Supplementary Figure S6 shows the effect of the combination of Olaparib and crizotinib in tumor growth
Supplementary Figure S7 shows the effect of knocking down ALK on the sensitivity of ovarian cancer cells to Olaparib/crizotinib combination
Data Availability Statement
The data generated in this study are available upon request from the corresponding author.