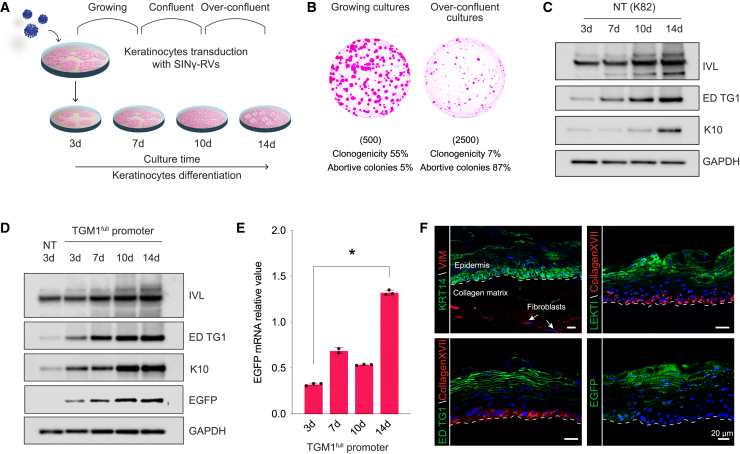
Figure 2.
The TGM1full promoter drives EGFP expression in primary differentiated human keratinocytes
(A) Schematic of the in vitro keratinocyte differentiation experimental design. (B) Representative colony-forming efficiency (CFE) of keratinocyte cultures in growing (left) (d7) and over-confluent (right) culture conditions (d14). The number of cells per dish plated in the CFE condition is indicated in brackets. Colonies were stained with Rhodamine B after 12 days of cultivation. (C) Western blot analysis of keratinocyte differentiation markers (IVL, endogenous TG1, and K10) in growing (3-day), confluent (7- to 10-day), and over-confluent (14-day) normal, healthy keratinocytes (K82). (D) Western blot analysis of keratinocyte differentiation markers (IVL, endogenous TG1, and K10) and EGFP in growing (3-day), confluent (7- to 10-day), and over-confluent (14-day) K82 keratinocytes transduced with a SINγ-RV carrying EGFP under the control of the TGM1full promoter. (E) Progressive (3- to 14-day) increase of EGFP mRNA during transgenic keratinocyte differentiation and stratification. GAPDH mRNA was used to normalize the RT-qPCR (∗p < 0.0001). (F) Representative immunofluorescence images of 7-μm-thick cryosections of 3D SEs obtained with TGM1full promoter-transduced keratinocytes, showing the expression of differentiation markers (endogenous TG1 and LEKTI) in the upper layers of the epidermis and collagen XVII and KRT14 as markers of the epidermal basal layer (n = 3, pictures are representative of what was observed in at least three independent samples or replicates). The EGFP fluorescent signal is properly restricted to the granular layer of the epidermis. White arrows indicate the presence of living fibroblasts inside the collagen matrix, expressing vimentin. A white dotted line marks the epidermal-dermal junction. DAPI (blue) stains nuclei. Scale bars, 20 μm. LEKTI, lympho-epithelial Kazal-type-related inhibitor; KRT14, cytokeratin 14; VIM, vimentin.