Abstract
Relaxin-2 is a peptide hormone with important roles in human cardiovascular and reproductive biology. Its ability to activate cellular responses such as vasodilation, angiogenesis, and anti-inflammatory and antifibrotic effects has led to significant interest in using relaxin-2 as a therapeutic for heart failure and several fibrotic conditions. However, recombinant relaxin-2 has a very short serum half-life, limiting its clinical applications. Here, we present protein engineering efforts targeting the relaxin-2 hormone in order to increase its serum half-life while maintaining its ability to activate the G protein-coupled receptor RXFP1. To achieve this, we optimized a fusion between relaxin-2 and an antibody Fc fragment, generating a version of the hormone with a circulating half-life of around 3 to 5 days in mice while retaining potent agonist activity at the RXFP1 receptor both in vitro and in vivo.
Keywords: relaxin, RXFP1, G protein-coupled receptor, protein engineering
Introduction
Relaxins are small protein hormones belonging to the insulin superfamily, exerting a variety of biological activities through the activation of G protein-coupled receptors.1 Within this family is relaxin-2, a reproductive hormone responsible for mediating many of the physiological changes of pregnancy through its cognate receptor, RXFP1.2 Relaxin-2 signaling through RXFP1 leads to vasodilation, angiogenesis, collagen degradation, and anti-inflammatory effects. In addition to relaxin-2’s role in pregnancy, these cellular responses also regulate the physiology of multiple organs in both sexes, including the liver, kidney, heart, lungs, and blood vessels.3,4 Activation of the pleiotropic effects downstream of RXFP1 can improve cardiac function and decrease fibrosis levels, which has generated interest in using relaxin-2 as a treatment for cardiovascular and fibrotic diseases.5−7
In animal models, recombinant relaxin-2 (serelaxin) has yielded promising results for the treatment of heart failure and fibrosis of the liver, lungs, kidneys, and joints.8−13 However, in large-scale clinical trials for acute heart failure, serelaxin treatment did not significantly decrease patient rehospitalization or mortality, although patients showed some short-term relief of symptoms such as dyspnea.14 One potential cause of these results is the short serelaxin administration time of 48 h, while patient data were collected up to 180 days after treatment. As a small protein hormone, serelaxin is rapidly cleared from circulation, with a serum half-life of less than 5 h.15 Therefore, the beneficial effects may be lost relatively quickly without continuous or repeated intravenous administration, limiting the use of serelaxin in many chronic conditions and introducing challenges to patient compliance.
The native relaxin-2 molecule has a two-polypeptide chain structure, with an A-chain and a B-chain connected by disulfide bonds, structurally similar to insulin.16 Protein engineering of the relaxin-2 molecule and small-molecule screening have each been explored to develop agonists of RXFP1 beyond the highly potent native relaxin-2 peptide, which has an EC50 of around 100 pM (Figure 1c and Table S1). Small-molecule library screens have proven to be challenging,17 and only one series of small-molecule agonists have been reported, with an EC50 of approximately 100 nM for the lead molecule, ML290.18,19 Optimizations of small-molecule potency, solubility, and stability starting from the ML290 scaffold led to the development of the RXFP1 allosteric agonist AZD5462.20,21 Versions of relaxin’s B-chain have also been produced and tested for activity at RXFP1. The B-chain alone showed reduced binding and cAMP signaling but maintained similar pERK potency in endogenously expressing RXFP1 cells.22,23 Further development of B-chain-only variants and the addition of lipid modifications resulted in multiple peptides being able to activate RXFP1 with improved potency and half-lives of up to 10 h in rats.24−28 Additionally, studies with mouse models of heart failure have tested fusions between relaxin-2 and an antibody Fc29 or a serum-albumin-binding VHH domain;30 however, limited details of protein sequence or engineering methods were reported.
Figure 1.
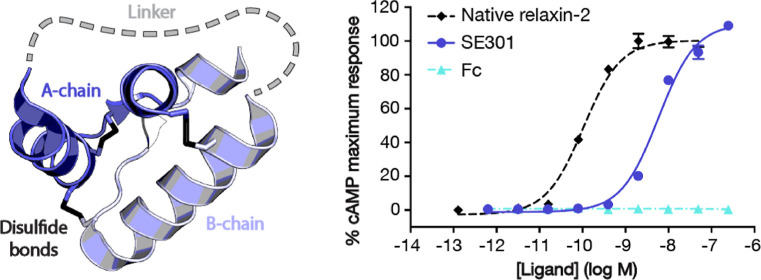
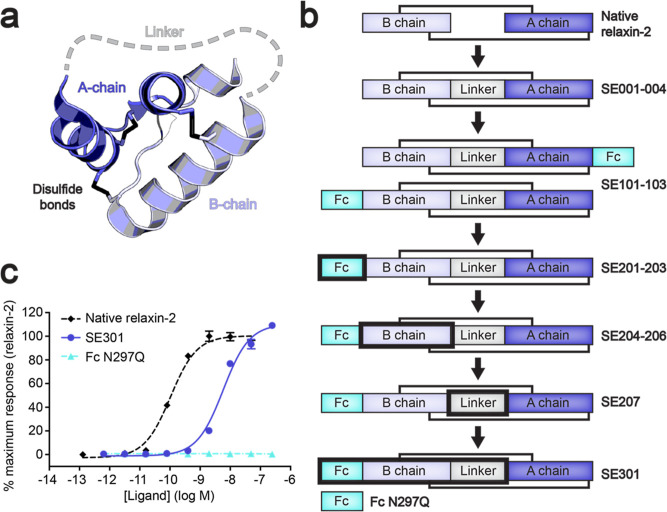
Engineering of an Fc–single-chain relaxin-2 fusion. (a) Diagram of single-chain relaxin-2 using the X-ray crystal structure of human relaxin-2 (PDB ID: 6RLX).16 (b) Overview of the rounds of optimization for the Fc–relaxin-2 fusion. The black box highlights regions that were optimized for each iteration. (c) CRE-SEAP Gs signaling assay data for human RXFP1 using SE301 and Fc N297Q compared to native relaxin-2. Data are normalized to the native relaxin-2 response and are the mean ± s.e.m. from technical triplicates.
Creating a fusion with an antibody Fc fragment is an established method of increasing the serum half-life of a protein of interest. Through neonatal Fc receptor (FcRN) binding, antibodies can avoid lysosomal degradation and be recycled back into circulation, resulting in a half-life of 2 to 3 weeks for most IgGs.31 Fc fusions to a protein of interest allow them to take advantage of the same recycling mechanism, conferring a long serum half-life to a protein that would otherwise be quickly cleared from circulation.32,33 Given the two-chain structure of the relaxin-2 hormone, a simple fusion with an Fc fragment is impossible. Moreover, any modifications to the relaxin-2 protein must be weighed against a reduction in signaling potency. In order to engineer an Fc–relaxin-2 fusion protein, we created a single-chain relaxin-2 molecule using a linker to connect the two peptide chains. Optimizations of the single-chain relaxin-2 sequence and fusion with human IgG1 Fc generated a molecule with a high biochemical stability and yield. The optimized fusion, SE301, maintains a high level of biological activity while gaining a long serum half-life. Moreover, it is straightforward and cost-effective to produce large quantities. Here, we describe the rational design of the SE301 molecule and the characterization of its in vitro and in vivo activity and pharmacokinetics profile.
Materials and Methods
Molecular Cloning
DNA encoding single-chain relaxins with an N-terminal hemagglutinin signal sequence and C-terminal 6× His-tag were cloned into the pcDNA-Zeo-tetO vector34 using PCR and NEBuilder HiFi DNA Assembly Mix (New England Biolabs). Fc fusion single-chain relaxins and the Fc N297Q negative control were cloned into pcDNA-Zeo-tetO with a N-terminal hemagglutinin signal sequence. For human and mouse RXFP1 and human RXFP2 expression constructs, receptors were cloned into pcDNA-Zeo-tetO with an N-terminal hemagglutinin signal sequence and a FLAG tag. Construct sequences are available in Table S7.
Protein Expression and Purification
His-Tagged Proteins
His-tagged relaxins were expressed as secreted proteins in Expi293F cells containing a stably integrated tetracycline repressor (Expi293F tetR, Thermo Fisher Scientific) grown in Expi293 medium (Thermo Fisher Scientific). Cells were transiently transfected with polyethylenimine; enhanced 24 h post-transfection with 0.4% glucose, 5 mM sodium butyrate, and 3 mM sodium valproic acid; and induced 48 h post-transfection with 5 mM sodium butyrate and 4 μg/mL doxycycline. The supernatant containing the single-chain relaxins was harvested from the cultures 5 days after induction by centrifugation at 4000g for 15 min at 4 °C.
To purify His-tagged single-chain relaxins, the supernatant was filtered with a glass fiber filter and loaded over Nickel Excel resin (GE Healthcare) equilibrated with 30 mM MES pH 6.5 and 300 mM sodium chloride. The resin was washed with 30 mM MES at pH 6.5, 300 mM sodium chloride, and 20 mM imidazole, and the protein was eluted with 30 mM MES at pH 6.5, 300 mM sodium chloride, and 500 mM imidazole. Ammonium sulfate was added to the eluted protein to 60% saturation and rotated at 4 °C for 1 h. The precipitated protein was centrifuged at 10,000g for 15 min at 4 °C, and the pellet was resuspended in 30 mM MES pH 6.5 and 300 mM sodium chloride. The resuspended protein was filtered by using a 0.1 μm pore size centrifugal filter and loaded onto a Sephadex S200 column (GE Healthcare) for size exclusion chromatography (SEC). Peak fractions were collected and concentrated with a centrifugal concentrator with a 3 kDa molecular weight cutoff. Purity of the proteins was assessed by SDS-PAGE gel. Aliquots were flash frozen in liquid nitrogen and stored at −80 °C.
Fc Fusion Proteins
Fc fusion single-chain relaxins and the Fc N297Q control were expressed in Expi293F tetR cells, as stated above. The supernatant containing the Fc fusions was harvested from the cultures 5 days after induction by centrifugation at 4000g for 15 min at 4 °C. The supernatant containing the Fc fusions was diluted 1:1 in 20 mM HEPES pH 7.5 and 150 mM sodium chloride (HBS) and loaded onto protein G resin (GE Healthcare) equilibrated with HBS. The resin was washed with HBS, and the protein was eluted with 100 mM glycine pH 2.5. The elution was neutralized to pH 7.5 with HEPES and dialyzed overnight in HBS. Samples prepared for mouse studies were dialyzed into phosphate buffered saline (PBS) at 4 °C. Elutions from large-scale cultures were diluted in 100 mM glycine at pH 2.5 prior to neutralization to avoid precipitation upon pH change. The dialyzed protein was concentrated with a centrifugal concentrator with a 3 kDa molecular weight cutoff. SDS-PAGE gels and analytical SEC were used to analyze proteins for purity and monodispersity. Proteins were aliquoted, flash frozen in liquid nitrogen, and stored at −80 °C.
Cellular Assays
cAMP Response Element-Secreted Embryonic Alkaline Phosphatase
Gs signaling was measured using an assay that indirectly detects cAMP production through transcription of the reporter enzyme secreted embryonic alkaline phosphatase (SEAP).35 In brief, clear 96-well plates were coated with 30 μL of 10 μg/mL poly-d-lysine and washed with PBS, and HEK293T cells (ATCC) were plated at 2.4 × 104 cells/well in Dulbecco’s modified Eagle’s medium (DMEM) with 10% (v/v) fetal bovine serum (FBS). The next day, the medium was replaced with 50 μL of serum-free DMEM. Lipofectamine 2000 (Thermo Fisher Scientific) was used to transfect cells at 70% confluency with 20 ng of cAMP response element (CRE)-SEAP reporter plasmid (Clontech) and 20 ng of receptor or empty vector pcDNA-Zeo-tetO DNA per well. Transfections were incubated for 5 h at 37 °C, and then the medium was replaced with 200 μL of serum-free DMEM plus ligand dilution curves. Twenty-four h later, the plates were incubated at 70 °C for 2 h. A solution of the SEAP substrate, 4-methylumbelliferyl phosphate (Sigma-Aldrich), was prepared at 120 μM in 2 M diethanolamine bicarbonate pH 10. The substrate solution was mixed with an equal volume (100 μL) of supernatant and incubated at room temperature (RT) for 15 min. An Envision 2103 multilabel reader (PerkinElmer) was used to measure fluorescence with an excitation wavelength of 360 nm and an emission wavelength of 449 nm. Signaling was calculated as a percentage of the native relaxin-2 response on either human or mouse RXPF1 and plotted using GraphPad Prism.
GloSensor
A real-time, live-cell signaling assay was used as a second method to measure the SE301 activation of Gs signaling through RXFP1. The assay was carried out as previously described.36 In brief, white, clear-bottomed 96-well plates were coated with poly-d-lysine and washed with PBS. HEK293T cells were then plated at 2.0 × 104 cells/well. The next day, cells were transfected with human RXFP1 pcDNA-Zeo-tetO and the GloSensor reporter plasmid using FuGENE (Promega) according to the manufacturer’s instructions. The cells were incubated for 24 h at 37 °C with 5% CO2. The next day, the medium was changed to 40 μL of CO2-independent medium (Thermo Fisher Scientific) with 10% (v/v) FBS and 2 mg/mL D-luciferin (GoldBio). The plates were then incubated for 2 h at RT in the dark. After 2 h, luminescence was measured before adding ligands using a SpectraMax M5 microplate reader with a 1 s integration time. A dilution series of SE301 or native relaxin-2 was added to the cells, and the luminescence measurement was repeated at 5, 10, 15, 20, 25, and 30 min after ligand addition. Signaling was calculated as a percentage of the native relaxin-2 response on human RXPF1 and plotted using GraphPad Prism.
Differential Scanning Fluorimetry
For differential scanning fluorimetry, SE301 was dialyzed overnight into PBS. Samples were prepared with 0.1 mg/mL SE301 or the dialysis buffer control and mixed with Protein Thermal Shift dye (Applied Biosystems) in a 1:100 ratio (v/v) of protein to dye in 96-well plates (Applied Biosystems). Differential scanning fluorimetry was carried out using the Life Technologies Quant Studio 6 with temperatures ranging from 25 to 99 °C, increasing by 3 °C per minute. Fluorescence was detected with 470 nm excitation and 586 nm emission filters. The fluorescence readings as a function of temperature were analyzed in the Protein Thermal Shift software (Applied Biosystems) by using the Boltzmann equation and plotted by using GraphPad Prism.
Flow Cytometry Binding Assay
To measure SE301 binding affinity, flow cytometry was used with Expi293F cells transfected with human RXFP1 or an empty pcDNA-Zeo-tetO vector. Expi293F tetR cells were grown in Expi293 media and transfected using FectoPRO (Polyplus) according to the manufacturer’s protocols. The cells were enhanced 24 h post-transfection with 0.4% glucose and induced 48 h post-transfection with 4 μg/mL doxycycline and 5 mM sodium butyrate. After 24 h of induction, cells were harvested by spinning at 200g for 5 min at 4 °C and washed once with HBS with 1% (v/v) FBS and 2 mM calcium chloride (binding buffer). Cells were plated into a V-bottom 96-well plate (Corning) at 100,000 cells/well and blocked by incubation in binding buffer for 30 min at 4 °C. After blocking, cells were centrifuged at 200g for 5 min at 4 °C, resuspended in 100 μL of binding buffer containing a dilution series of SE301 or Fc N297Q, and incubated for 1 h at 4 °C. Cells were then centrifuged at 200g for 5 min at 4 °C, washed twice with 200 μL of binding buffer, and resuspended in 100 μL of binding buffer containing 100 nM M1 anti-FLAG antibody labeled with Alexa Fluor 488 and Alexa Fluor 647 antihuman IgG Fc (BioLegend) diluted 1:100 (v/v). Cells were incubated in secondary antibodies for 30 min at 4 °C, washed once with 200 μL of binding buffer, and resuspended in 100 μL of binding buffer for flow cytometry. Samples were analyzed on a BD Accuri C6 flow cytometer (BD Biosciences) and gated according to plots of FSC-A/SCA-A, FSC-A/FSC-H, and receptor expression according to Alexa Fluor 488 M1 anti-FLAG antibody binding. Approximately 1000 events/sample were collected from cells expressing receptors for human RXFP1-transfected cells or post-FSC-A/FSC-H gating for empty vector-transfected cells. Mean fluorescence intensities for Alexa Fluor 647 antihuman IgG Fc binding were plotted and analyzed in GraphPad Prism.
In Silico Immunogenicity Analysis
A computational analysis of immunogenicity was performed through Abzena Ltd. The sequence of SE301 was analyzed as 9 amino acid peptides in increments of one amino acid. Each peptide was scored for possible MHC class II binding and homology to known T cell epitopes.
Mouse Pharmacokinetics Study
SE301 was prepared in sterile PBS at 10 mg/mL using the methods described above. A pharmacokinetics study in mice was conducted through WuXi AppTec Ltd. to determine the serum half-life of the SE301 molecule. For the study, male CD-1 mice were used with intraperitoneal injections (IP) of SE301 at one of three doses: 1, 5, or 50 mg/kg. Nine mice were used in the pharmacokinetics study, three per dose of SE301, and each was between 7 and 10 weeks of age and weighed between 29 and 40 g. The IP injection of SE301 was administered via hypogastric regions, and blood samples were taken from the mice before dosing and 2, 24, 72, and 168 h postdosing. At each time point, at least 0.6 mL of blood was collected from each animal. The blood samples were stored at RT for about 30 min and then centrifuged at 2500g for 15 min at 4 °C. After centrifugation, the serum was collected, an aliquot was taken for analysis, and the samples were frozen over dry ice and stored at −60 °C or lower. An ELISA was conducted to detect human IgG1 Fc to determine the amount of SE301 per sample. Serum SE301 concentration versus time point data were plotted in the WinNonlin software program to derive pharmacokinetics parameters.
Mouse Hemodynamics Study
Animal experiments carried out for this study were handled according to approved protocols and animal welfare regulations mandated by the Institutional Animal Care and Use Committee of the Duke University Medical Center. Eight to 12 week-old C57BL/6J wild-type mice of both sexes were used for this study. Mice were anesthetized with ketamine (100 mg/kg) and xylazine (2.5 mg/kg), and bilateral vagotomy was performed. The right carotid artery was cannulated with a 1.4 French (0.46 mm) high-fidelity micromanometer catheter (Millar SPR-671) and advanced into the left ventricle to measure ventricular pressure and heart rate.37 Basal heart rate and ventricular pressure were recorded at steady state after catheter insertion (2–3 min after insertion). Graded doses of Fc N297Q (20, 40, 80, and 400 μg) or SE301 (25, 50, 100, and 500 μg) were administered at 10 min intervals by intravenous injection through a left jugular vein. Hemodynamics were monitored continuously and recorded at steady state (10 min after each injection). Data analysis was performed using LabChart 8 software (ADInstruments).37
Results and Discussion
Engineering of a Single-Chain Relaxin-2
The native relaxin-2 hormone is translated as a single polypeptide chain. In order of sequence, the prohormone consists of a B-chain of 29 residues, a connecting C-chain of 108 residues, and an A-chain of 24 residues. After translation, the C-chain of prorelaxin-2 is cleaved by proteolytic digestion. The resulting protein is the mature form of native relaxin-2, in which the B-chain and A-chain are separate alpha-helical peptides connected by two interchain disulfide bonds.38
The first methods of producing native relaxin-2 utilized chemical synthesis of the two separate chains in reduced forms, followed by an oxidative step to form the disulfide bonds.39,40 Methods using recombinant DNA technology were able to improve the yields for relaxin-2 using a construct containing a “mini-C” peptide linker between the B and A chains. These methods utilized the published X-ray crystal structure of relaxin-2 to design a shortened C-chain length of 13 residues that would still be long enough to connect the two chains.16,41 These relaxin proteins were expressed in Escherichia coli, purified from inclusion bodies, and refolded. The “mini-C” linkers were then removed by protease digestion, resulting in a recombinant form of the native relaxin-2 hormone.41
To generate a mature single-chain version of relaxin-2, we first tested a linker originally used as a cleavable “mini-C” peptide for relaxin-3, another member of the relaxin hormone family.42 We expressed and purified single-chain relaxin-2 with an N-terminal hemagglutinin signal sequence as a His-tagged secreted protein in mammalian cells without removing the “mini-C” linker (Figure 1a,b). The expression protocol was purposefully chosen to determine if a less labor-intensive method could be used to produce properly folded and biologically active relaxin-2. The protein showed a monodisperse size exclusion profile and high purity as determined by Coomassie-stained SDS-PAGE gels (Figure S1a,b). To determine whether our single-chain relaxin-2 maintained activity at RXFP1, the protein was tested using a cell-based assay for Gs signaling. The single-chain relaxin-2 (SE001) maintained subnM potency at human RXFP1, illustrating the feasibility of generating single-polypeptide relaxin-2 molecules with high biological activity from mammalian expression systems (Figure S1c).
In later rounds of protein engineering, the sequence of the “mini-C” linker was further optimized (Figure 1b). The first linker, Asp-Ala-Ala-Ser-Ser-His-Ser-His-Ser-Ser-Ala-Arg, contained several Ser residues and His residues that were removed from the redesigned sequence. Ser residues were changed to remove potential sites of O-linked glycosylation, and His residues were changed to prevent any pH-dependent changes in binding affinity. After redesign, the new linker consisted of the sequence Asp-Ala-Ala-Gly-Ala-Asn-Ala-Asn-Ala-Gly-Ala-Arg (SE207).
Optimization of Fc–Single-Chain Relaxin-2 Fusions
The biologically active single-chain relaxin-2 created an opportunity to alter several of the protein’s properties through engineering additional fusions. The main purpose of these modifications was to lengthen the short serum half-life of the small relaxin-2 protein in order to generate a long-half-life agonist of the RXFP1 receptor. To accomplish this, we tested fusions of a human IgG1 Fc antibody fragment to either the N- or C-terminus of single-chain relaxin-2 (SE101–103, Figure 1b). Gs signaling assays showed that N-terminal fusions maintained higher signaling potency at human RXFP1 than that of C-terminal fusions (8.3 nM vs 49.4 nM, Figure S2a). These proteins also had increased purification yields, up to 400-fold higher than those of the initial construct SE001.
At this stage, a mutation was introduced into the human IgG1 Fc fragment for all further constructs. The mutation N297Q removes a glycosylation site from the Fc fragment that is important for IgG1 effector functions. As a result, the abilities of IgG1 Fc to activate complement and antibody-dependent cellular cytotoxicity are ablated in the N297Q mutant.43 The fusion site between Fc N297Q and the N-terminus of single-chain relaxin-2 was the next region to be optimized (Figure 1b). The Fc fragment had initially been fused to the single-chain relaxin-2 N-terminus by a linker of Gly-Gly-Ser repeats. Fusions with a shorter three-residue linker length achieved higher signaling potency than that of a 12-residue Gly-Gly-Ser linker (SE101–103, Figure S2a). Sequences for the 3-residue linker were then varied by constructing linkers with the sequences Ala-Ala-Ala and Pro-Pro-Pro in addition to the Gly-Gly-Ser linker (SE201–203). Each of these iterations maintained similar signaling potency and biochemical properties (Figure S2b); therefore, Gly-Gly-Ser was chosen for the final linker sequence.
Finally, several mutations were introduced to the B-chain of single-chain relaxin-2 to improve its biochemical properties (SE204–206, Figure 1b). Met4 and Met25 were mutated to avoid residues that were prone to oxidation in the relaxin molecule. The Met residues were mutated to Lys according to the sequences of relaxin-2 orthologues, offering an idea of what residues may be tolerated in that position. Additionally, Trp28 was mutated to Ala in order to remove a residue that may increase the protein polyreactivity. These three mutations were tolerated in the Fc–relaxin-2 fusions, having similar or better signaling potency than the native sequence (Figure S2c). In a docking model of the interactions between relaxin-2 and the ligand-binding ectodomain of the RXFP1 receptor,36 the residues targeted for mutagenesis are not positioned near the binding interface, explaining the lack of disruption to relaxin-2 activity (Figure S3).
Biochemical and Functional Characterization of SE301
The optimized features of the Fc–relaxin-2 fusions were combined in the final molecule, SE301. This molecule contained, in order of sequence, the hemagglutinin signal sequence, the Fc N297Q fragment, a 3 residue Gly-Gly-Ser linker, and single-chain relaxin-2 with the redesigned “mini-C” linker and the Met4 to Lys, Met25 to Lys, and Trp28 to Ala mutations to the B-chain (Figure 1b). In silico immunogenicity analysis of the SE301 sequence was used to predict possible MHC class II binding peptides and their homology to known T cell epitopes. The computational analysis concluded that no major liabilities were created by changes made to the native relaxin sequence.
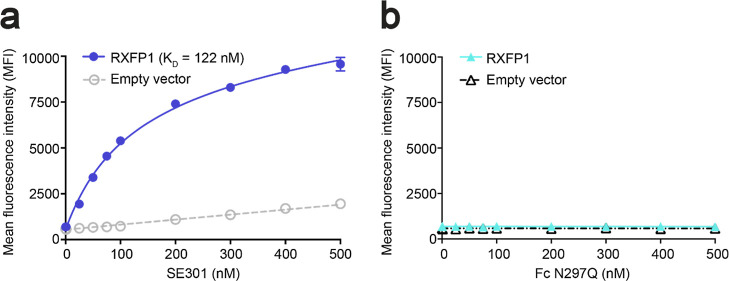
When tested in a Gs signaling assay, SE301 had an EC50 of 5.8 nM at human RXFP1, with an Emax at approximately 100% of native relaxin-2 (Figure 1c). While maintaining the strong activation of RXFP1, SE301 also showed very little off-target activity at the related RXFP2 receptor (Figure S4a). The measured signaling potency of SE301 at human RXFP1 was confirmed with a secondary Gs signaling assay method, which showed close agreement with our initial experiments (EC50 of 7.1 nM, Figure S5). Next, the binding affinity for SE301 was tested using a flow cytometry assay with mammalian cells transfected with RXFP1 or an empty vector. SE301’s KD for human RXFP1 was determined to be 122 nM (Figure 2a), while a control molecule of the Fc N297Q fragment alone showed no binding (Figure 2b) or signaling (Figure 1c) with RXFP1-expressing cells.
Figure 2.
Determination of SE301 binding affinity for RXFP1. (a,b) Flow cytometry binding data for SE301 (a) and Fc N297Q (b) using human RXFP1 and empty vector-transfected Expi293F cells. The KD for SE301 at human RXFP1 was calculated to be 122 ± 36 nM. Data are the mean ± s.e.m. from technical duplicates.
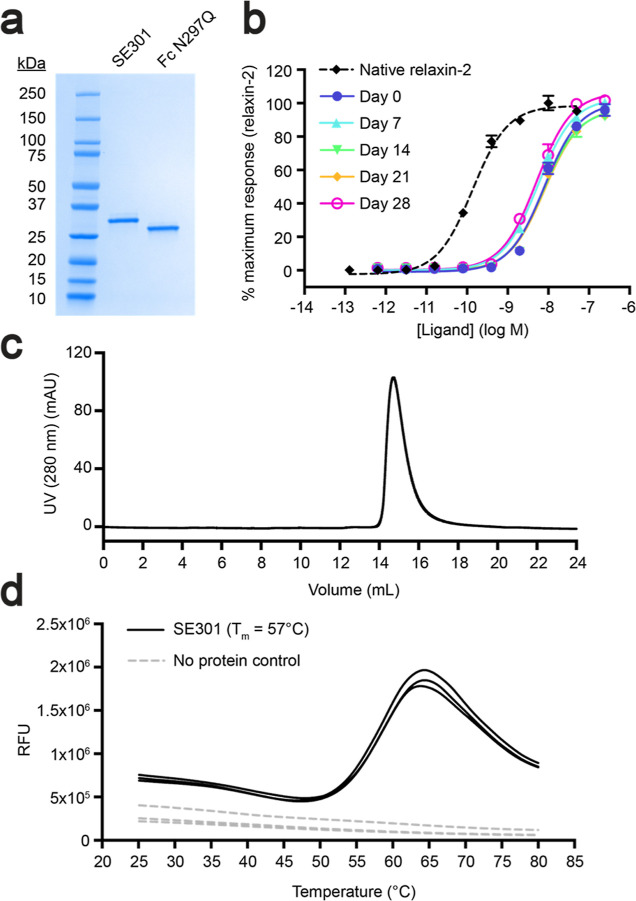
Additional biochemical studies for SE301 utilized differential scanning fluorimetry to determine a melting temperature (Tm) of 57 °C (Figure 3d). Furthermore, SE301 showed high stability at RT, maintaining a similar signaling efficacy and potency after 4 weeks of incubation (Figure 3b). The protocols to produce SE301 as a secreted protein from mammalian cells utilized straightforward expression and purification methods, with a yield of approximately 150 mg per 1 L of culture (see Methods). Collectively, the results from characterization studies showed that SE301 is a potent RXFP1 agonist with high purity, monodispersity, and yield (Figure 3a,c).
Figure 3.
Characterization of SE301 purity, monodispersity, and stability. (a) Coomassie-stained SDS-PAGE gel of SE301 and Fc N297Q. (b) CRE-SEAP Gs signaling testing the activity of SE301 at human RXFP1 after incubations at RT for 0, 7, 14, 21, and 28 days, showing that SE301 retains activity after 4 weeks at RT. Data are normalized to the native relaxin-2 response at human RXFP1 and are the mean ± s.e.m. from technical triplicates. (c) Size exclusion profile for SE301 shows a monodisperse peak. (d) Differential scanning fluorimetry for SE301 determined its melting temperature to be 57 °C.
Pharmacokinetics of SE301
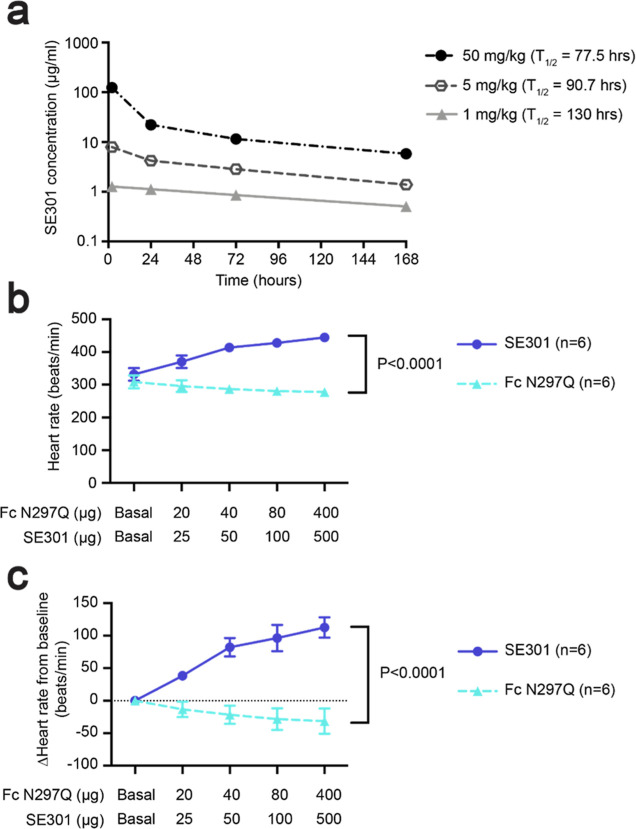
After the biochemical and functional characterization of the SE301 molecule, we conducted experiments to determine the serum half-life of SE301 in vivo. To answer this question, we conducted a pharmacokinetics study in mice using a single injection of SE301 at one of three doses: 1, 5, or 50 mg/kg. Serum samples were taken before injection and then at 2, 24, 72, and 168 h postinjection. To determine the concentration of SE301 remaining in circulation at each time point, the serum samples were analyzed by an ELISA detecting the human IgG1 Fc of SE301. Based on concentrations interpolated from the ELISA data, the serum half-life was calculated to be between 77.5 and 130 h, depending on the dose of SE301 (Figure 4a). These results approach the 6 to 8 day serum half-life of IgGs in mice,44 showing that the Fc fragment was able to confer a longer circulating half-life to SE301.
Figure 4.
Pharmacokinetics and in vivo hemodynamics. (a) Pharmacokinetics study of SE301 in mice used a single intraperitoneal injection and measured the SE301 serum concentration by ELISA from samples taken before injection and 2, 24, 72, and 168 h postinjection. The serum half-life for SE301 was calculated to be between 77.5 and 130 h. Data are the mean ± s.e.m. and n = 3 for each dose. (b,c) Mouse in vivo hemodynamics study for IV administration of SE301 or Fc N297Q. Heart rate was monitored upon increasing doses of SE301 or Fc N297Q. Plots show either the measured heart rate (b) or the value calculated as a change from the baseline heart rate (c). Data are the mean ± s.e.m. and n = 6, p < 0.0001.
SE301 Shows In Vivo Activity
After establishing the long serum half-life of SE301, we conducted a study to determine the activity of the molecule at the RXFP1 receptor in vivo. In rodents, RXFP1 expressed in the atria of the heart causes a positive chronotropic effect upon relaxin-2 treatment, with an increase in heart rate of around 130 beats per minute (bpm) above baseline.45,46 The heart rate changes in response to relaxin-2 directly result from activation of RXFP1 and are not an effect of catecholamine release.46 The chronotropic effects do not translate to humans because RXFP1 is not expressed in the atria, and relaxin-2 administration has been shown to have no effect on heart rate in clinical trials.47 Although it does not directly translate to any human disease, the effect on rodent heart rate represents a short-term experiment that tested RXFP1 agonist activity in vivo. Therefore, we chose to carry out a mouse hemodynamics study to test SE301 in advance of longer-timeline studies with animal models of human disease.
First, the ability of our engineered human relaxin-2 fusion to activate mouse RXFP1 was tested using cell-based Gs signaling. The assay determined an EC50 of 8.6 nM for SE301 at mouse RXFP1, establishing the utility of mouse models for our in vivo studies (Figure S4b). In the hemodynamics study, mice were anesthetized, and the heart rate of the left ventricle was monitored. Increasing doses of the Fc N297Q-alone control molecule or SE301 were administered by intravenous injection at 10 min intervals. Mice showed a dose-dependent increase in heart rate in response to SE301, increasing from around 330 bpm at baseline to 445 bpm after injection with 500 μg of SE301 (Figure 4b,c). In contrast, mice showed no increase in heart rate in response to Fc N297Q. Together, these data established the ability of SE301 to activate the RXFP1 receptor in vivo.
Conclusions
Here, we describe each step in the design of a long-half-life RXFP1 agonist. Through applying protein engineering strategies to the hormone relaxin-2, we generated a potent agonist of the RXFP1 receptor with a long serum half-life as well as high yield, purity, and biochemical stability. Our general approach should be extensible to other relaxin family peptide hormones, although optimal mutations, linker lengths, and fusion sites will likely vary due to the different modes of ligand recognition among the relaxin receptors.
In mouse studies with our final Fc–relaxin-2 fusion, SE301 confirmed an extended half-life of around 3 to 5 days and established its in vivo activity at RXFP1. In a mouse hemodynamics study, SE301 increased heart rate in a manner comparable with the native relaxin-2 peptide.46 Future work will test the efficacy of SE301 in animal models of cardiovascular or fibrotic conditions, in which treatment with the native relaxin-2 peptide has proven promising. In those experiments, recombinant versions of native relaxin-2 are typically administered continuously,9−11,13 similar to the intravenous infusions of the relaxin-2 peptide used in clinical trials.14 Given its extended half-life, our engineered Fc–relaxin-2 has the potential to achieve a similar improvement in disease phenotypes through weekly or biweekly administration via subcutaneous injections. The results of these experiments will potentially provide a path toward accessing the beneficial biological effects of relaxin-2 for a wider range of indications.
Acknowledgments
We would like to thank Dr. Kelly L. Arnett for assistance with differential scanning fluorimetry measurements and the Center for Macromolecular Interactions at Harvard Medical School, where differential scanning fluorimetry experiments were performed. This work was supported by National Institutes of Health grants HL056687 and HL075443 to H.A.R., an NIH Ruth L. Kirschstein predoctoral fellowship (F31 GM128233) to S.C.E., a Damon Runyon postdoctoral fellowship (no. DRG: 2489-23 awarded to J.O.-O.), and a Blavatnik Biomedical Accelerator grant from Harvard Medical School to A.C.K.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.molpharmaceut.4c00368.
Data from the purification and characterization of single-chain relaxin-2, signaling assay data from the optimizations of Fc–relaxin-2 fusions, docking model highlighting sites of relaxin-2 engineering, SE301 signaling activity at human RXFP2 and mouse RXFP1, SE301 versus native relaxin-2 activity in an orthogonal cAMP assay, signaling assay EC50 and Emax data, and construct sequences (PDF)
The authors declare the following competing financial interest(s): A.C.K. and S.C.E. are inventors on patent application PCT/US2021/031260 for engineered single-chain relaxin proteins. A.C.K. is a cofounder and consultant for Tectonic Therapeutic and Seismic Therapeutic, and for the Institute for Protein Innovation, a nonprofit research institute.
Supplementary Material
References
- Bathgate R. A. D.; Halls M. L.; van der Westhuizen E. T.; Callander G. E.; Kocan M.; Summers R. J. Relaxin family peptides and their receptors. Physiol. Rev. 2013, 93, 405–480. 10.1152/physrev.00001.2012. [DOI] [PubMed] [Google Scholar]
- Hsu S. Y.; Nakabayashi K.; Nishi S.; Kumagai J.; Kudo M.; Sherwood O. D.; Hsueh A. J. W. Activation of orphan receptors by the hormone relaxin. Science 2002, 295, 671–674. 10.1126/science.1065654. [DOI] [PubMed] [Google Scholar]
- Samuel C. S.; Royce S. G.; Hewitson T. D.; Denton K. M.; Cooney T. E.; Bennett R. G. Anti-fibrotic actions of relaxin. Br. J. Pharmacol. 2017, 174, 962–976. 10.1111/bph.13529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarwar M.; Du X. J.; Dschietzig T. B.; Summers R. J. The actions of relaxin on the human cardiovascular system. Br. J. Pharmacol. 2017, 174, 933–949. 10.1111/bph.13523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devarakonda T.; Salloum F. N. Heart disease and relaxin: new actions for an old hormone. Trends Endocrinol. Metab. 2018, 29, 338–348. 10.1016/j.tem.2018.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng H. H.; Shen M.; Samuel C. S.; Schlossmann J.; Bennett R. G. Relaxin and extracellular matrix remodeling: mechanisms and signaling pathways. Mol. Cell. Endocrinol. 2019, 487, 59–65. 10.1016/j.mce.2019.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai A. J.; Konieczko E. M.; Bennett R. G.; Samuel C. S.; Royce S. G. Relaxin and fibrosis: emerging targets, challenges, and future directions. Mol. Cell. Endocrinol. 2019, 487, 66–74. 10.1016/j.mce.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blessing W. A.; Okajima S. M.; Cubria M. B.; Villa-Camacho J. C.; Perez-Viloria M.; Williamson P. M.; Sabogal A. N.; Suarez S.; Ang L. H.; White S.; et al. Intraarticular injection of relaxin-2 alleviates shoulder arthrofibrosis. Proc. Natl. Acad. Sci. U.S.A. 2019, 116, 12183–12192. 10.1073/pnas.1900355116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett R. G.; Heimann D. G.; Singh S.; Simpson R. L.; Tuma D. J. Relaxin decreases the severity of established hepatic fibrosis in mice. Liver Int. 2014, 34, 416–426. 10.1111/liv.12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber S. L.; Mirochnik Y.; Brecklin C. S.; Unemori E. N.; Singh A. K.; Slobodskoy L.; Grove B. H.; Arruda J. A.; Dunea G. Relaxin decreases renal interstitial fibrosis and slows progression of renal disease. Kidney Int. 2001, 59, 876–882. 10.1046/j.1523-1755.2001.059003876.x. [DOI] [PubMed] [Google Scholar]
- Unemori E. N.; Pickford L. B.; Salles A. L.; Piercy C. E.; Grove B. H.; Erikson M. E.; Amento E. P. Relaxin induces an extracellular matrix-degrading phenotype in human lung fibroblasts in vitro and inhibits lung fibrosis in a murine model in vivo. J. Clin. Invest. 1996, 98, 2739–2745. 10.1172/JCI119099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle Raleigh J.; et al. Reperfusion therapy with recombinant human relaxin-2 (Serelaxin) attenuates myocardial infarct size and NLRP3 inflammasome following ischemia/reperfusion injury via eNOS-dependent mechanism. Cardiovasc. Res. 2017, 113, 609–619. 10.1093/cvr/cvw246. [DOI] [PubMed] [Google Scholar]
- Beiert T.; Tiyerili V.; Knappe V.; Effelsberg V.; Linhart M.; Stöckigt F.; Klein S.; Schierwagen R.; Trebicka J.; Nickenig G.; et al. Relaxin reduces susceptibility to post-infarct atrial fibrillation in mice due to anti-fibrotic and anti-inflammatory properties. Biochem. Biophys. Res. Commun. 2017, 490, 643–649. 10.1016/j.bbrc.2017.06.091. [DOI] [PubMed] [Google Scholar]
- Metra M.; Teerlink J. R.; Cotter G.; Davison B. A.; Felker G. M.; Filippatos G.; Greenberg B. H.; Pang P. S.; Ponikowski P.; Voors A. A.; et al. Effects of serelaxin in patients with acute heart failure. N. Engl. J. Med. 2019, 381, 716–726. 10.1056/NEJMoa1801291. [DOI] [PubMed] [Google Scholar]
- Chen S. A.; Perlman A. J.; Spanski N.; Peterson C. M.; Sanders S. W.; Jaffe R.; Martin M.; Yalcinkaya T.; Cefalo R. C.; Chescheir N. C.; et al. The pharmacokinetics of recombinant human relaxin in nonpregnant women after intravenous, intravaginal, and intracervical administration. Pharm. Res. 1993, 10, 834–838. 10.1023/A:1018901009062. [DOI] [PubMed] [Google Scholar]
- Eigenbrot C.; et al. X-ray structure of human relaxin at 1.5 A: Comparison to insulin and implications for receptor binding determinants. J. Mol. Biol. 1991, 221, 15–21. 10.1016/0022-2836(91)90796-9. [DOI] [PubMed] [Google Scholar]
- McBride A.; Hoy A. M.; Bamford M. J.; Mossakowska D. E.; Ruediger M. P.; Griggs J.; Desai S.; Simpson K.; Caballero-Hernandez I.; Iredale J. P.; et al. In search of a small molecule agonist of the relaxin receptor RXFP1 for the treatment of liver fibrosis. Sci. Rep. 2017, 7, 10806. 10.1038/s41598-017-10521-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J.; Huang Z.; Chen C. Z.; Agoulnik I. U.; Southall N.; Hu X.; Jones R. E.; Ferrer M.; Zheng W.; Agoulnik A. I.; et al. Identification and optimization of small-molecule agonists of the human relaxin hormone receptor RXFP1. Nat. Commun. 2013, 4, 1953. 10.1038/ncomms2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson K. J.; Xiao J.; Chen C. Z.; Huang Z.; Agoulnik I. U.; Ferrer M.; Southall N.; Hu X.; Zheng W.; Xu X.; et al. Optimization of the first small-molecule relaxin/insulin-like family peptide receptor (RXFP1) agonists: activation results in an antifibrotic gene expression profile. Eur. J. Med. Chem. 2018, 156, 79–92. 10.1016/j.ejmech.2018.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granberg K. L.; Sakamaki S.; Fuchigami R.; Niwa Y.; Fujio M.; Kato H.; Bergström F.; Larsson N.; Persson M.; Villar I. C.; et al. Identification of novel series of potent and selective relaxin family peptide receptor 1 (RXFP1) agonists. J. Med. Chem. 2024, 67, 4442–4462. 10.1021/acs.jmedchem.3c02183. [DOI] [PubMed] [Google Scholar]
- Granberg K. L.; Sakamaki S.; Larsson N.; Bergström F.; Fuchigami R.; Niwa Y.; Ryberg E.; Backmark A.; Kato H.; Miyazaki S.; et al. Discovery of clinical candidate AZD5462, a selective oral allosteric RXFP1 agonist for treatment of heart failure. J. Med. Chem. 2024, 67, 4419–4441. 10.1021/acs.jmedchem.3c02184. [DOI] [PubMed] [Google Scholar]
- Hossain M. A.; Kocan M.; Yao S. T.; Royce S. G.; Nair V. B.; Siwek C.; Patil N. A.; Harrison I. P.; Rosengren K. J.; Selemidis S.; et al. A single-chain derivative of the relaxin hormone is a functionally selective agonist of the G protein-coupled receptor, RXFP1. Chem. Sci. 2016, 7, 3805–3819. 10.1039/C5SC04754D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam F.; Gaspari T. A.; Kemp-Harper B. K.; Low E.; Aw A.; Ferens D.; Spizzo I.; Jefferis A. M.; Praveen P.; Widdop R. E.; et al. The single-chain relaxin mimetic, B7–33, maintains the cardioprotective effects of relaxin and more rapidly reduces left ventricular fibrosis compared to perindopril in an experimental model of cardiomyopathy. Biomed. Pharmacother. 2023, 160, 114370. 10.1016/j.biopha.2023.114370. [DOI] [PubMed] [Google Scholar]
- Mallart S.; Ingenito R.; Bianchi E.; Bresciani A.; Esposito S.; Gallo M.; Magotti P.; Monteagudo E.; Orsatti L.; Roversi D.; et al. Identification of potent and long-acting single-chain peptide mimetics of human relaxin-2 for cardiovascular diseases. J. Med. Chem. 2021, 64, 2139–2150. 10.1021/acs.jmedchem.0c01533. [DOI] [PubMed] [Google Scholar]
- Poirier B.; Pasquier O.; Chenede X.; Corbier A.; Prigent P.; Azam A.; Bernard C.; Guillotel M.; Gillot F.; Riva L.; et al. R2R01: a long-acting single-chain peptide agonist of RXFP1 for renal and cardiovascular diseases. Br. J. Pharmacol. 2024, 181, 1993–2011. 10.1111/bph.16338. [DOI] [PubMed] [Google Scholar]
- Illiano S.; Poirier B.; Minoletti C.; Pasquier O.; Riva L.; Chenede X.; Menguy I.; Guillotel M.; Prigent P.; Le Claire S.; et al. Characterization of a new potent and long-lasting single chain peptide agonist of RXFP1 in cells and in vivo translational models. Sci. Rep. 2022, 12 (1), 20435. 10.1038/s41598-022-24716-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praveen P.; Wang C.; Handley T. N. G.; Wu H.; Samuel C. S.; Bathgate R. A. D.; Hossain M. A. A lipidated single-B-chain derivative of relaxin exhibits improved in vitro serum stability without altering activity. Int. J. Mol. Sci. 2023, 24, 6616. 10.3390/ijms24076616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handley T. N. G.; Praveen P.; Tailhades J.; Wu H.; Bathgate R. A. D.; Hossain M. A. Further developments towards a minimal potent derivative of human relaxin-2. Int. J. Mol. Sci. 2023, 24, 12670. 10.3390/ijms241612670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J.; Hao W.; Fillmore N.; Ma H.; Springer D.; Yu Z.; Sadowska A.; Garcia A.; Chen R.; Muniz-Medina V.; et al. Human relaxin-2 fusion protein treatment prevents and reverses isoproterenol-induced hypertrophy and fibrosis in mouse heart. J. Am. Heart Assoc. 2019, 8, e013465 10.1161/jaha.119.013465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdino P.; Lee S. L.; Cooper F. N.; Cottle S. R.; Grealish P. F.; Hu C. C.; Meyer C. M.; Lin J.; Copeland V.; Porter G.; et al. Development of a long-acting relaxin analogue, LY3540378, for treatment of chronic heart failure. Br. J. Pharmacol. 2023, 180, 1965–1980. 10.1111/bph.16055. [DOI] [PubMed] [Google Scholar]
- Mankarious S.; Lee M.; Fischer S.; Pyun K. H.; Ochs H. D.; Oxelius V. A.; Wedgwood R. J. The half-lives of IgG subclasses and specific antibodies in patients with primary immunodeficiency who are receiving intravenously administered immunoglobulin. J. Lab. Clin. Med. 1988, 112, 634–640. [PubMed] [Google Scholar]
- Ko S.; Jo M.; Jung S. T. Recent achievements and challenges in prolonging the serum half-lives of therapeutic IgG antibodies through Fc engineering. BioDrugs 2021, 35, 147–157. 10.1007/s40259-021-00471-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czajkowsky D. M.; Hu J.; Shao Z.; Pleass R. J. Fc-fusion proteins: new developments and future perspectives. EMBO Mol. Med. 2012, 4, 1015–1028. 10.1002/emmm.201201379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staus D. P.; Wingler L. M.; Choi M.; Pani B.; Manglik A.; Kruse A. C.; Lefkowitz R. J. Sortase ligation enables homogeneous GPCR phosphorylation to reveal diversity in β-arrestin coupling. Proc. Natl. Acad. Sci. U.S.A. 2018, 115, 3834–3839. 10.1073/pnas.1722336115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberles S. D.; Buck L. B. A second class of chemosensory receptors in the olfactory epithelium. Nature 2006, 442, 645–650. 10.1038/nature05066. [DOI] [PubMed] [Google Scholar]
- Erlandson S. C.; Rawson S.; Osei-Owusu J.; Brock K. P.; Liu X.; Paulo J. A.; Mintseris J.; Gygi S. P.; Marks D. S.; Cong X.; et al. The relaxin receptor RXFP1 signals through a mechanism of autoinhibition. Nat. Chem. Biol. 2023, 19, 1013–1021. 10.1038/s41589-023-01321-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon C.; Staus D. P.; Wingler L. M.; Wang J.; Skiba M. A.; Elgeti M.; Hubbell W. L.; Rockman H. A.; Kruse A. C.; Lefkowitz R. J. Synthetic nanobodies as angiotensin receptor blockers. Proc. Natl. Acad. Sci. U.S.A. 2020, 117, 20284–20291. 10.1073/pnas.2009029117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson P.; John M.; Crawford R.; Haralambidis J.; Scanlon D.; Gorman J.; Tregear G.; Shine J.; Niall H. Relaxin gene expression in human ovaries and the predicted structure of a human preprorelaxin by analysis of cDNA clones. EMBO J. 1984, 3, 2333–2339. 10.1002/j.1460-2075.1984.tb02135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büllesbach E.; Schwabe C. Total synthesis of human relaxin and human relaxin derivatives by solid-phase peptide synthesis and site-directed chain combination. J. Biol. Chem. 1991, 266, 10754–10761. 10.1016/S0021-9258(18)99082-4. [DOI] [PubMed] [Google Scholar]
- Tregear G. W.; Wade J. D.. Chemistry, Synthesis, and processing of relaxin. In Progress in Relaxin Research—The Proceedings of the Second International Congress on the Hormone Relaxin; World Scientific Publishing Co Pte Ltd, 1994; pp 31–44. [Google Scholar]
- Vandlen R.; Winslow J.; Moffat B.; Rinderknecht E.. Human relaxin: purification, characterization and production of recombinant relaxins for structure function studies. In Progress in Relaxin Research—The Proceedings of the Second International Congress on the Hormone Relaxin; World Scientific Publishing Co Pte Ltd, 1994; pp 59–72. [Google Scholar]
- Luo X.; Liu Y. L.; Layfield S.; Shao X. X.; Bathgate R. A.; Wade J. D.; Guo Z. Y. A simple approach for the preparation of mature human relaxin-3. Peptides 2010, 31, 2083–2088. 10.1016/j.peptides.2010.07.022. [DOI] [PubMed] [Google Scholar]
- Jefferis R. Recombinant antibody therapeutics: the impact of glycosylation on mechanisms of action. Trends Pharmacol. Sci. 2009, 30, 356–362. 10.1016/j.tips.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Vieira P.; Rajewsky K. The half-lives of serum immunoglobulins in adult mice. Eur. J. Immunol. 1988, 18, 313–316. 10.1002/eji.1830180221. [DOI] [PubMed] [Google Scholar]
- Ward D. G.; Thomas G. R.; Cronin M. J. Relaxin increases rat heart rate by a direct action on the cardiac atrium. Biochem. Biophys. Res. Commun. 1992, 186, 999–1005. 10.1016/0006-291X(92)90845-C. [DOI] [PubMed] [Google Scholar]
- Kakouris H.; Eddie L. W.; Summers R. J. Cardiac effects of relaxin in rats. Lancet 1992, 339, 1076–1078. 10.1016/0140-6736(92)90665-P. [DOI] [PubMed] [Google Scholar]
- Ponikowski P.; Mitrovic V.; Ruda M.; Fernandez A.; Voors A. A.; Vishnevsky A.; Cotter G.; Milo O.; Laessing U.; Zhang Y.; et al. A randomized, double-blind, placebo-controlled, multicentre study to assess haemodynamic effects of serelaxin in patients with acute heart failure. Eur. Heart J. 2014, 35, 431–441. 10.1093/eurheartj/eht459. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.