Abstract
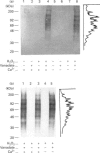
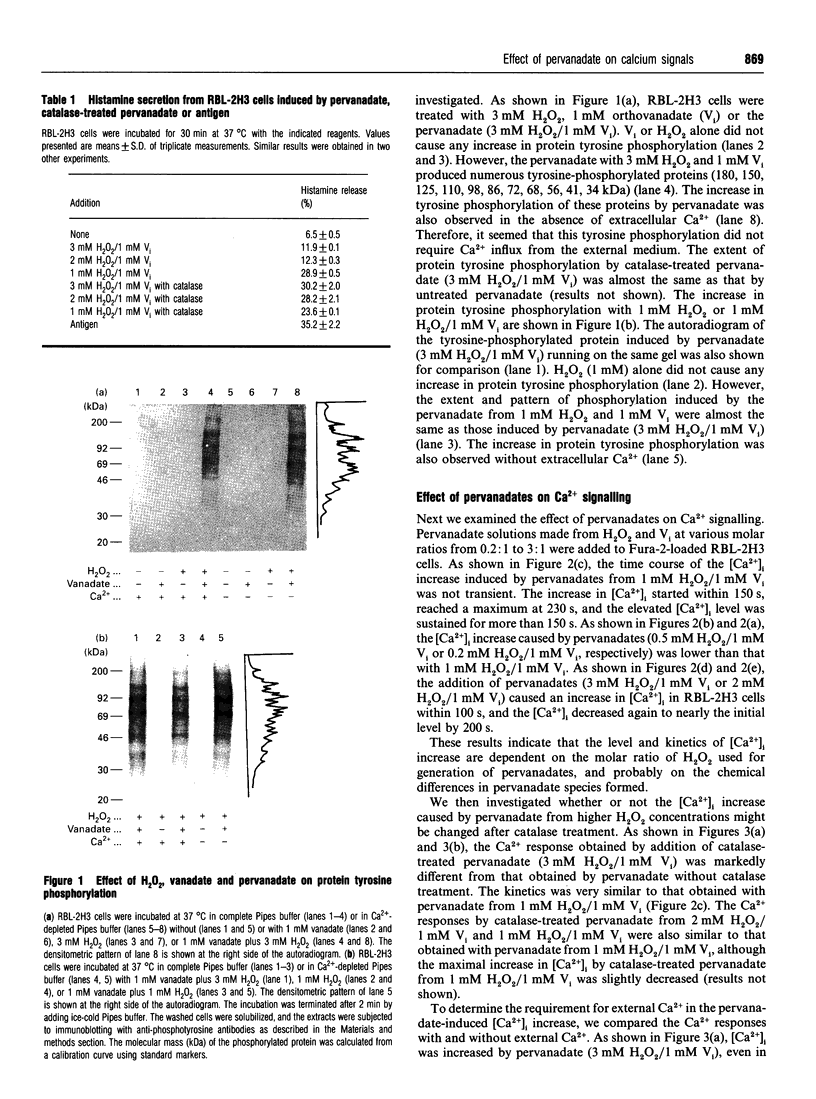
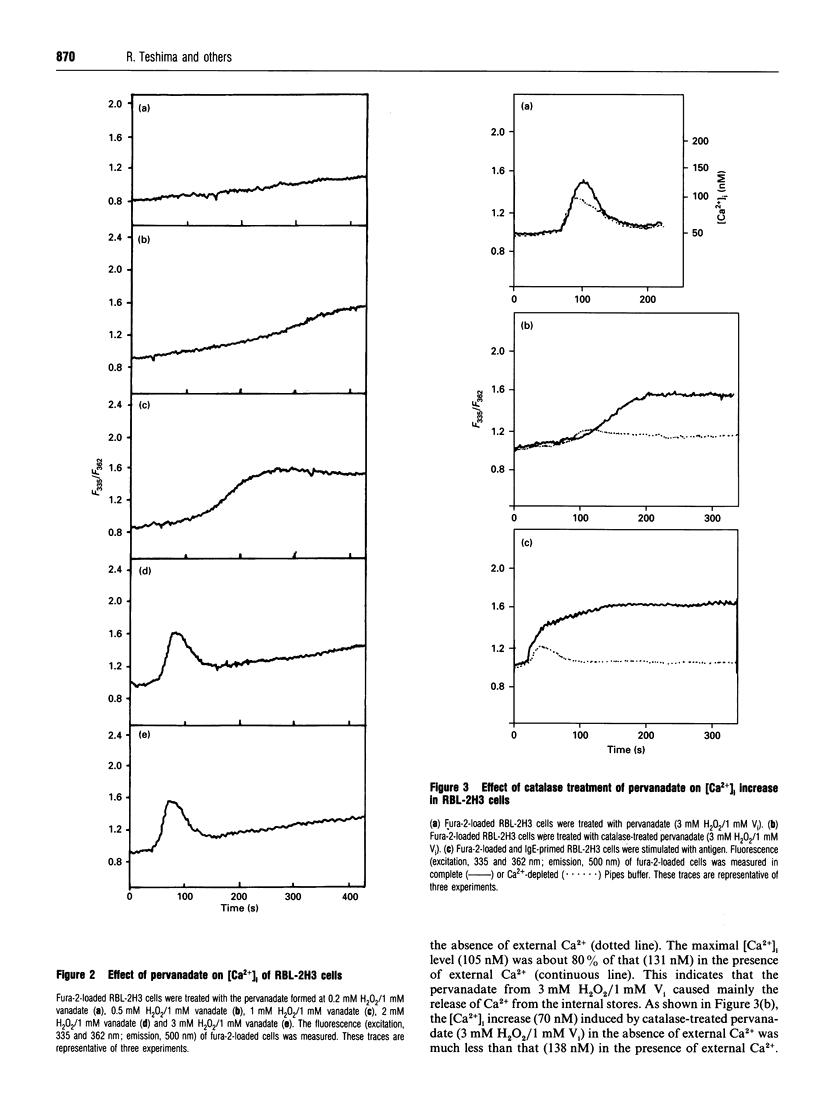
We examined the effect of pervanadate on the activation of rat basophilic leukaemia (RBL-2H3) cells. The pervanadate, generated from a combination of H2O2 and vanadate (Vi), induced concomitantly protein tyrosine phosphorylation, formation of inositol 1,4,5-trisphosphate (IP3), an increase in [Ca2+]i, and histamine secretion in RBL-2H3 cells. These effects were clearly dependent on the ratio of H2O2/Vi. The secretion of histamine, IP3 formation, and sustained increase in [Ca2+]i were effectively induced by treatment of the cells with the pervanadate produced from 1 mM H2O2 and 1 mM Vi. These effects mimic the stimulatory effects of an antigen (dinitrophenylated BSA) on Ca2+ signals, histamine secretion and morphological changes. Protein tyrosine phosphorylation, formation of IP3 and transient increase in [Ca2+]i were markedly induced by the pervanadate produced from 3 mM H2O2 and 1 mM Vi. However, histamine secretion induced by the pervanadate was very low. After the pervanadate from 3 mM H2O2 and 1 mM Vi was treated with catalase, it was able to induce the [Ca2+]i increase and histamine secretion as much as the antigen did. This indicates that pervanadate from a lower H2O2 concentration (1 mM H2O2/1 mM Vi) and catalase-treated pervanadate from a higher H2O2 concentration (3 mM H2O2/1 mM Vi) are able to mimic the activity that was caused by cross-linking of IgE receptors with antigen. The present results also demonstrate that protein tyrosine phosphorylation seems to have a crucial role in Ca2+ entry from the external medium, and that a sustained [Ca2+]i increase is an important step for histamine secretion in RBL-2H3 cells.
Full text
PDF

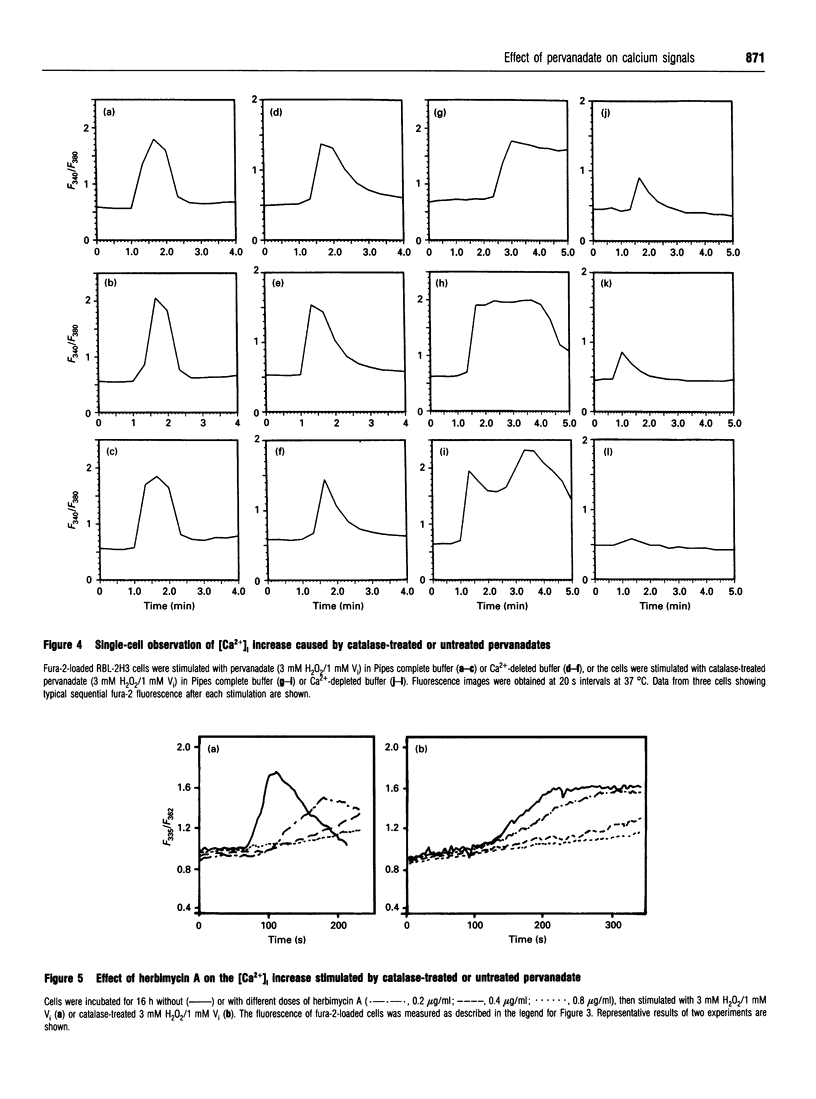



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barsumian E. L., Isersky C., Petrino M. G., Siraganian R. P. IgE-induced histamine release from rat basophilic leukemia cell lines: isolation of releasing and nonreleasing clones. Eur J Immunol. 1981 Apr;11(4):317–323. doi: 10.1002/eji.1830110410. [DOI] [PubMed] [Google Scholar]
- Beemon K., Ryden T., McNelly E. A. Transformation by avian sarcoma viruses leads to phosphorylation of multiple cellular proteins on tyrosine residues. J Virol. 1982 May;42(2):742–747. doi: 10.1128/jvi.42.2.742-747.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhamou M., Gutkind J. S., Robbins K. C., Siraganian R. P. Tyrosine phosphorylation coupled to IgE receptor-mediated signal transduction and histamine release. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5327–5330. doi: 10.1073/pnas.87.14.5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao W., Liu H., Olson M. S. Effect of orthovanadate on tyrosine phosphorylation of P120 GTPase-activating protein in rat liver macrophages (Kupffer cells). Biochem Biophys Res Commun. 1993 Feb 26;191(1):55–60. doi: 10.1006/bbrc.1993.1183. [DOI] [PubMed] [Google Scholar]
- Eiseman E., Bolen J. B. Engagement of the high-affinity IgE receptor activates src protein-related tyrosine kinases. Nature. 1992 Jan 2;355(6355):78–80. doi: 10.1038/355078a0. [DOI] [PubMed] [Google Scholar]
- Fantus I. G., Kadota S., Deragon G., Foster B., Posner B. I. Pervanadate [peroxide(s) of vanadate] mimics insulin action in rat adipocytes via activation of the insulin receptor tyrosine kinase. Biochemistry. 1989 Oct 31;28(22):8864–8871. doi: 10.1021/bi00448a027. [DOI] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Zokas L., Kamps M. P. Monoclonal antibodies to phosphotyrosine. J Immunol Methods. 1988 May 9;109(2):277–285. doi: 10.1016/0022-1759(88)90253-0. [DOI] [PubMed] [Google Scholar]
- Heffetz D., Bushkin I., Dror R., Zick Y. The insulinomimetic agents H2O2 and vanadate stimulate protein tyrosine phosphorylation in intact cells. J Biol Chem. 1990 Feb 15;265(5):2896–2902. [PubMed] [Google Scholar]
- Iino M., Endo M. Calcium-dependent immediate feedback control of inositol 1,4,5-triphosphate-induced Ca2+ release. Nature. 1992 Nov 5;360(6399):76–78. doi: 10.1038/360076a0. [DOI] [PubMed] [Google Scholar]
- Kadota S., Fantus I. G., Deragon G., Guyda H. J., Hersh B., Posner B. I. Peroxide(s) of vanadium: a novel and potent insulin-mimetic agent which activates the insulin receptor kinase. Biochem Biophys Res Commun. 1987 Aug 31;147(1):259–266. doi: 10.1016/s0006-291x(87)80115-8. [DOI] [PubMed] [Google Scholar]
- Kadota S., Fantus I. G., Deragon G., Guyda H. J., Posner B. I. Stimulation of insulin-like growth factor II receptor binding and insulin receptor kinase activity in rat adipocytes. Effects of vanadate and H2O2. J Biol Chem. 1987 Jun 15;262(17):8252–8256. [PubMed] [Google Scholar]
- Kato K., Nakanishi M., Arata Y., Teshima R., Terao T., Miyamoto H. Calcium influx in a single rat basophilic leukemia cell as revealed with a digital imaging fluorescence microscope. J Biochem. 1987 Jul;102(1):1–4. doi: 10.1093/oxfordjournals.jbchem.a122020. [DOI] [PubMed] [Google Scholar]
- Kawakami T., Inagaki N., Takei M., Fukamachi H., Coggeshall K. M., Ishizaka K., Ishizaka T. Tyrosine phosphorylation is required for mast cell activation by Fc epsilon RI cross-linking. J Immunol. 1992 Jun 1;148(11):3513–3519. [PubMed] [Google Scholar]
- Klarlund J. K. Transformation of cells by an inhibitor of phosphatases acting on phosphotyrosine in proteins. Cell. 1985 Jul;41(3):707–717. doi: 10.1016/s0092-8674(85)80051-9. [DOI] [PubMed] [Google Scholar]
- Kumada T., Miyata H., Nozawa Y. Involvement of tyrosine phosphorylation in IgE receptor-mediated phospholipase D activation in rat basophilic leukemia (RBL-2H3) cells. Biochem Biophys Res Commun. 1993 Mar 31;191(3):1363–1368. doi: 10.1006/bbrc.1993.1367. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leis J. F., Kaplan N. O. An acid phosphatase in the plasma membranes of human astrocytoma showing marked specificity toward phosphotyrosine protein. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6507–6511. doi: 10.1073/pnas.79.21.6507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. A., Austen K. F. Mediation of local homeostasis and inflammation by leukotrienes and other mast cell-dependent compounds. Nature. 1981 Sep 10;293(5828):103–108. doi: 10.1038/293103a0. [DOI] [PubMed] [Google Scholar]
- Margolis B., Hu P., Katzav S., Li W., Oliver J. M., Ullrich A., Weiss A., Schlessinger J. Tyrosine phosphorylation of vav proto-oncogene product containing SH2 domain and transcription factor motifs. Nature. 1992 Mar 5;356(6364):71–74. doi: 10.1038/356071a0. [DOI] [PubMed] [Google Scholar]
- O'Shea J. J., McVicar D. W., Bailey T. L., Burns C., Smyth M. J. Activation of human peripheral blood T lymphocytes by pharmacological induction of protein-tyrosine phosphorylation. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10306–10310. doi: 10.1073/pnas.89.21.10306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver J. M., Seagrave J., Stump R. F., Pfeiffer J. R., Deanin G. G. Signal transduction and cellular response in RBL-2H3 mast cells. Prog Allergy. 1988;42:185–245. [PubMed] [Google Scholar]
- Park D. J., Min H. K., Rhee S. G. IgE-induced tyrosine phosphorylation of phospholipase C-gamma 1 in rat basophilic leukemia cells. J Biol Chem. 1991 Dec 25;266(36):24237–24240. [PubMed] [Google Scholar]
- Santini F., Beaven M. A. Tyrosine phosphorylation of a mitogen-activated protein kinase-like protein occurs at a late step in exocytosis. Studies with tyrosine phosphatase inhibitors and various secretagogues in rat RBL-2H3 cells. J Biol Chem. 1993 Oct 25;268(30):22716–22722. [PubMed] [Google Scholar]
- Secrist J. P., Burns L. A., Karnitz L., Koretzky G. A., Abraham R. T. Stimulatory effects of the protein tyrosine phosphatase inhibitor, pervanadate, on T-cell activation events. J Biol Chem. 1993 Mar 15;268(8):5886–5893. [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Beemon K., Eckhart W. Evidence that the phosphorylation of tyrosine is essential for cellular transformation by Rous sarcoma virus. Cell. 1980 Jul;20(3):807–816. doi: 10.1016/0092-8674(80)90327-x. [DOI] [PubMed] [Google Scholar]
- Stephan V., Benhamou M., Gutkind J. S., Robbins K. C., Siraganian R. P. Fc epsilon RI-induced protein tyrosine phosphorylation of pp72 in rat basophilic leukemia cells (RBL-2H3). Evidence for a novel signal transduction pathway unrelated to G protein activation and phosphatidylinositol hydrolysis. J Biol Chem. 1992 Mar 15;267(8):5434–5441. [PubMed] [Google Scholar]
- Swarup G., Cohen S., Garbers D. L. Inhibition of membrane phosphotyrosyl-protein phosphatase activity by vanadate. Biochem Biophys Res Commun. 1982 Aug;107(3):1104–1109. doi: 10.1016/0006-291x(82)90635-0. [DOI] [PubMed] [Google Scholar]
- Teshima R., Ikebuchi H., Sawada J., Furuno T., Nakanishi M., Terao T. Effects of herbimycin A and ST638 on Fc epsilon receptor-mediated histamine release and Ca2+ signals in rat basophilic leukemia (RBL-2H3) cells. Biochim Biophys Acta. 1994 Mar 10;1221(1):37–46. doi: 10.1016/0167-4889(94)90213-5. [DOI] [PubMed] [Google Scholar]
- Teshima R., Ikebuchi H., Terao T., Miyagawa T., Arata Y., Nakanishi M. A single cell observation of staurosporine effect on the Ca2+ signals in rat basophilic leukemia cells. FEBS Lett. 1990 Sep 17;270(1-2):115–118. doi: 10.1016/0014-5793(90)81247-l. [DOI] [PubMed] [Google Scholar]
- Teshima R., Ikebuchi H., Terao T., Nakanishi M. The effect of staurosporine on Ca2+ signals in rat basophilic leukemia (RBL-2H3) cells. Chem Pharm Bull (Tokyo) 1991 Mar;39(3):747–751. doi: 10.1248/cpb.39.747. [DOI] [PubMed] [Google Scholar]
- Uehara Y., Fukazawa H., Murakami Y., Mizuno S. Irreversible inhibition of v-src tyrosine kinase activity by herbimycin A and its abrogation by sulfhydryl compounds. Biochem Biophys Res Commun. 1989 Sep 15;163(2):803–809. doi: 10.1016/0006-291x(89)92293-6. [DOI] [PubMed] [Google Scholar]
- Zick Y., Sagi-Eisenberg R. A combination of H2O2 and vanadate concomitantly stimulates protein tyrosine phosphorylation and polyphosphoinositide breakdown in different cell lines. Biochemistry. 1990 Nov 6;29(44):10240–10245. doi: 10.1021/bi00496a013. [DOI] [PubMed] [Google Scholar]