Fig. 1. Activity-guided fractionation identifies PTER as a N-acetyltaurine hydrolase.
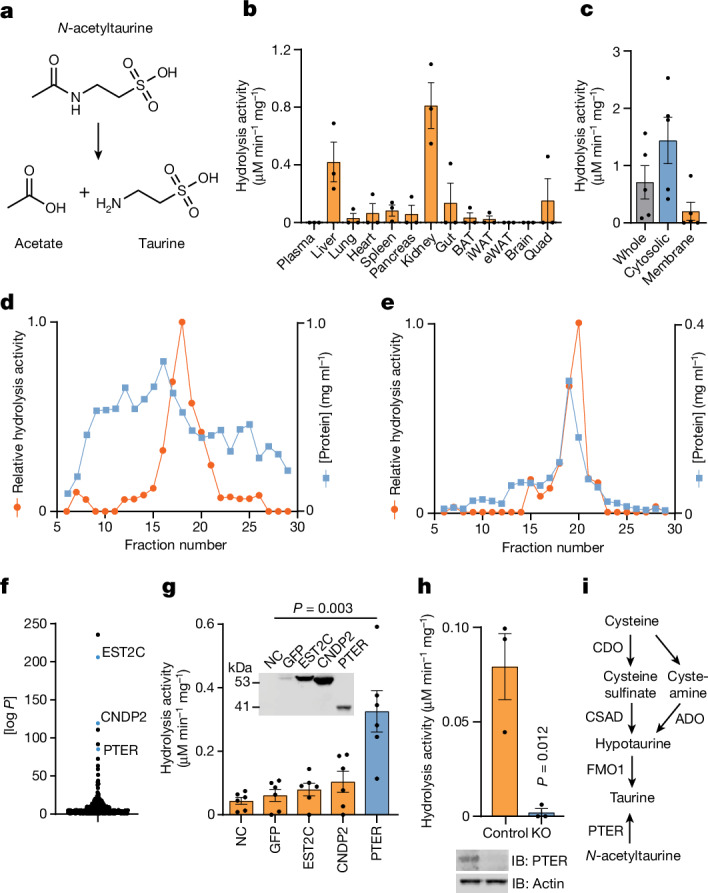
a, Schematic showing the conversion of N-acetyltaurine to acetate and taurine. b, N-acetyltaurine hydrolysis activity of the indicated mouse whole-tissue homogenate. Tissue samples were collected from 10–14-week-old male C57BL/6J mice. Reactions were performed using 100 µg tissue homogenates at 37 °C for 1 h with 100 µM N-acetyltaurine. N = 3 per group. BAT, brown adipose tissue; eWAT, epididymal white adipose tissue; iWAT, inguinal white adipose tissue; Quad, quadriceps muscle. c, N-acetyltaurine hydrolysis activity in the indicated fraction of total kidney lysate. Reactions were performed as in b. N = 5 per group. d,e, Relative N-acetyltaurine hydrolysis activity and protein concentrations of the indicated fraction following anion-exchange chromatography (d) or size-exclusion chromatography (e). N = 1 per data point. f, Byonic P values of proteins identified in fraction 20 following size-exclusion chromatography. g,h, N-acetyltaurine hydrolysis activity from HEK293T cell lysates after transfection with the indicated plasmids (g, N = 6 per group) or from control or PTER KO cell lysates (h, N = 3 per group). Reactions were performed as in b. Western blots in g and h used an anti-Flag antibody of HEK293T cell lysates 2 days after the indicated transfection (g) or anti-PTER antibody in WT and PTER KO cells (h). IB, immunoblot. i, Schematic of revised taurine metabolic pathway showing the role of PTER as a N-acetyltaurine hydrolase. ADO, cysteamine deoxygenate; CDO, cysteine dioxygenase; CSAD, cysteine sulfinic acid decarboxylase; FMO1, flavin-containing monooxygenase 1. For b, c, g and h, data are shown as the mean ± s.e.m. For h, the loading control was performed on the same blot. In g and h, P values were calculated from two-tailed unpaired t-tests and not adjusted for multiple comparisons. All experiments were repeated twice and similar results were obtained.
