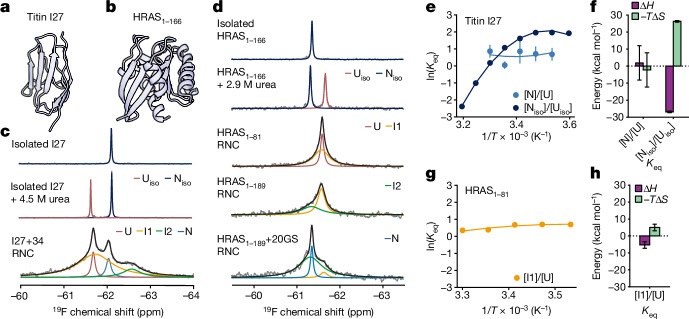
Fig. 4. Co-translational folding intermediates of titin I27 and HRAS are stabilized on the ribosome.
a,b, Crystal structures of titin I27 (Protein Data Bank (PDB): 1TIT) (a) and HRAS (PDB: 4Q21) (b). c, 19F NMR spectra of titin I27, tfmF-labelled at position 14, off the ribosome (isolated), isolated I27 in urea, and I27 tethered with a 34-residue linker to the ribosome (I27+34 RNC) recorded at 298 K. d, 19F NMR spectra of the HRAS G-domain (residues 1–166), tfmF-labelled at position 32, off the ribosome (isolated), in urea and HRAS on the ribosome arrested at 3 different lengths and recorded at 298 K. HRAS1–81, HRAS1–189 and HRAS1–189+20GS correspond to residues 1–81 of HRAS, full-length HRAS and full-length HRAS with an additional 20-residue poly(glycine-serine) linker, respectively, each tethered to the ribosome by the arrest-enhanced SecM motif. e,g, Nonlinear fits to a modified Gibbs–Helmholtz equation (Methods) of the equilibrium constants (mean ± s.e.m. propagated from NMR lineshape fits) of titin I27 folding off and on the ribosome (e) and the HRAS1-81 RNC (g) measured by 19F NMR. f, Thermodynamic parameters determined from the nonlinear fit (mean ± s.d., T = 298 K) of the temperature dependence of I27 folding. The heat capacity of folding (ΔCp,N-U) obtained for the isolated and ribosome-bound protein are −2.0 ± 0.1 and +0.6 ± 1.7 kcal mol−1 K−1, respectively. The heat capacity of the isolated protein is similar to the literature value reported for the wild-type variant28 (−1.4 kcal mol−1). h, Thermodynamic parameters determined from the nonlinear fit (mean ± s.d., T = 298 K) of the temperature dependence of folding for HRAS1–81. The heat capacity of folding obtained from the fit is −0.4 ± 0.3 kcal mol−1 K−1.