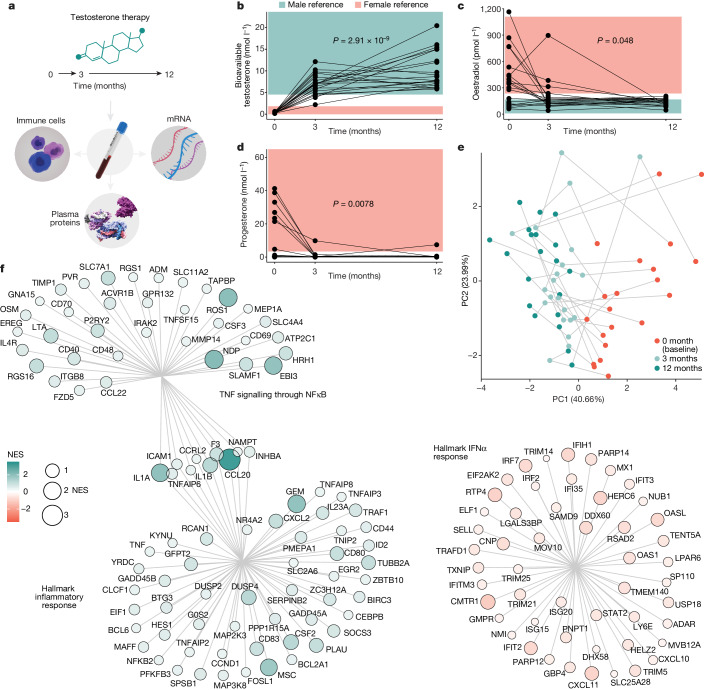
Fig. 1. Immunological investigation in individuals undergoing gender-affirming testosterone therapy.
a, Systems-level assessment of blood immune system in individuals assigned female sex at birth (trans men) in blood samples collected at baseline, and after 3 and 12 months of oral testosterone therapy (n = 23). b–d, Sex hormone concentrations measured in serum samples (n = 66) using liquid chromatography with tandem mass spectrometry in a single experiment and shown in relation to female (pink) and male (blue) reference ranges before and during testosterone therapy. Kruskal–Wallis tests (5% false discovery rate (FDR) corrected) for bioavailable testosterone (b), oestradiol (c) and progesterone (d). e, PCA on the basis of nine sex hormones, first two principal components (PC1 and PC2; percentage variance explained) and sample points coloured by sample timepoint. f, Bulk RNA-seq from whole blood samples (n = 60) and differently expressed mRNA transcripts analysed by normalized enrichment scores (NES) for Hallmark pathways. Hallmark IFNα responses decrease after 12 months of testosterone treatment, TNF signalling through NFκB and Hallmark inflammatory responses increased after 12 months of testosterone treatment as compared with baseline.