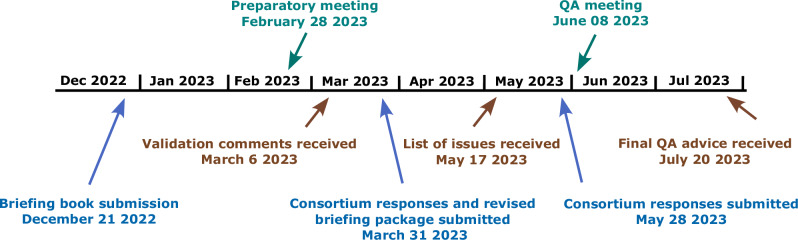
Fig. 2. Qualification advice process and timelines.
Overall, the advice period lasted 7 months, which was preceded by the compilation of the briefing book (5 months). Validation comments from the European Medicines Agency (EMA) were received 3 months after submission. After submitting a revised version of the briefing book, the consortium received a list of issues that were addressed in a written response as well as an online meeting. The final Qualification Advice was received 7 months after the briefing book submission. Submissions by the consortium are shown in blue, CHMP responses in brown and meetings in green.