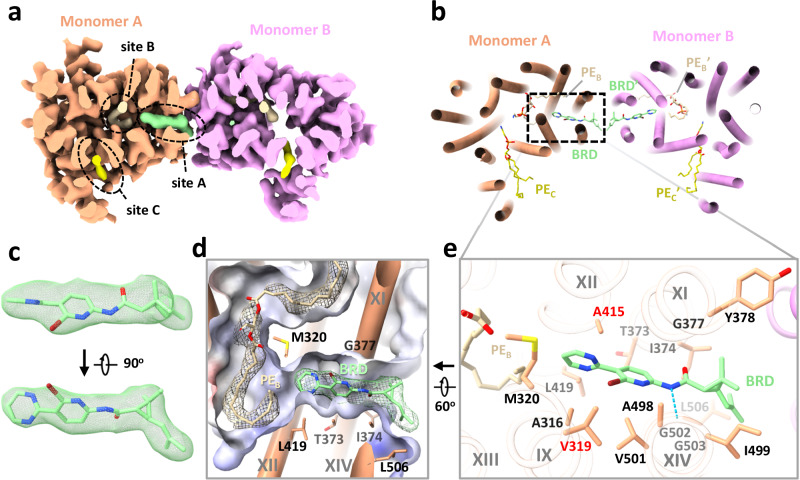
Fig. 3. Structure of MtEfpA bound to the inhibitor BRD-8000.3.
a Top view of BRD-8000.3 density (light green) at the lipid binding site A in one MtEfpA monomer (light orange). b Cartoon model of MtEfpA bound to BRD-8000.3 (BRD, light green) and two phosphatidylethanolamine (PE) molecules (wheat at site B and yellow at site C). c Superimposition of the BRD-8000.3 model and density (5σ). d Electrostatic surface of the BRD-8000.3 binding pocket. e Residues of MtEfpA (light orange) interacting with BRD-8000.3 (light green) within 4 Å. The hydrogen bond between A489’s O3 of MtEfpA and N9 of BRD-8000.3 is shown in a blue dashed line. Resistant mutation residues are labeled in red.