The complex sits in a general position. Each NiII ion has an N4Cl2 coordination sphere. Weak hydrogen bonding exists between three of the amino groups and the chloride ions of an adjacent molecule. Chains of molecules, linked by the hydrogen bonding and short Cl⋯Cl contacts, are well separated by the 3-methoxyaniline ligands.
Keywords: nickel chloride, 3-methoxyaniline, NiN4Cl2 coordination, crystal structure
Abstract
The reaction of nickel(II) chloride with 3-methoxyaniline yielded dichloridotetrakis(3-methoxyaniline)nickel(II), [NiCl2(C7H9NO)4], as yellow crystals. The NiII ion is pseudo-octahedral with the chloride ions trans to each other. The four 3-methoxyaniline ligands differ primarily due to different conformations about the Ni—N bond, which also affect the hydrogen bonding. Intermolecular N—H⋯ Cl hydrogen bonds and short Cl⋯Cl contacts between molecules link them into chains parallel to the b axis.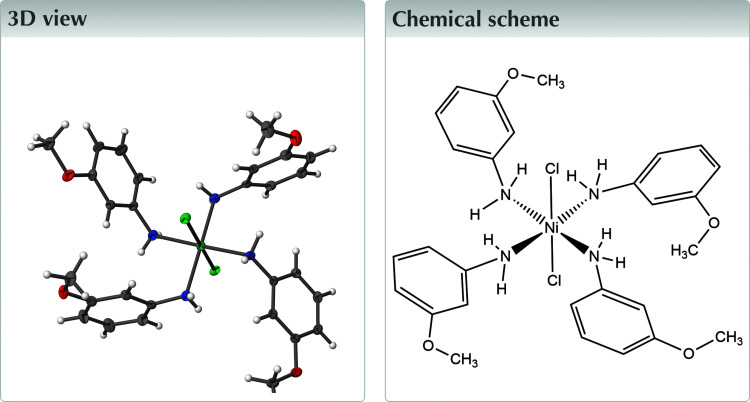
Structure description
The structures of binary transition-metal halide complexes of aniline are varied and have been known for nearly two decades, since the report of CoCl2(aniline)2 by Burrow et al. (1997 ▸). Structures for compounds of the formula MX2(aniline)2, where M is a transition metal, are known for trans-square planar (SP) Pd (Chen et al., 2002 ▸) and Cu (Low et al., 2013 ▸), and tetrahedral (Td) Zn (Khan et al., 2010 ▸; Ejaz et al., 2009 ▸; Rademeyer et al., 2004 ▸) and Cd (Costin-Hogan et al., 2008 ▸). Structures of first row transition-metal (FTM) complexes with the same general formula, FTMX2(sub-aniline)2 are known for substituents such as o-methyl (SP: Daniliuc et al., 2023 ▸), p-methyl (Td: Chellali et al., 2019 ▸), p-ethyl (Td: Govindaraj et al., 2015 ▸; Td: Harmouzi et al., 2017 ▸), p-acetyl (Td and SP: Macek et al., 2023 ▸; SP, Nemec et al., 2020 ▸), p-bromo (Td: Subashini et al., 2012a ▸; Td, Li: 2023 ▸), p-chloro (Td: Chellali et al., 2019 ▸), p-fluoro (Td: Subashini et al., 2012b ▸), o-methoxy, m-methoxy and p-methoxy (Td: Kupko et al., 2020 ▸; Td: Amani, 2018 ▸) and p-carboxylic acid (Td: Rademeyer et al., 2010 ▸; SP: Guedes et al., 2011 ▸). Only slightly less common, but particularly favored by NiII, are those structures of the formula FTMX2(sub-aniline)2(solvent)2, which include solvents such as water (Macek et al., 2023 ▸; Meehan et al., 2021 ▸) methanol (Meehan et al., 2021 ▸), ethanol (Meehan et al., 2021 ▸; Clegg & Martin, 2007 ▸) and acetonitrile (Fawcett et al., 2005 ▸); all are trans-pseudooctahedral (Oh). A smaller number of structures have been reported with aniline and substituted aniline ligands of the formula FTMX2(sub-aniline)4, which include the trans-Oh complexes NiCl2(p-methylaniline)4 and NiBr2(p-methylaniline)4 (Meehan et al., 2021 ▸) and NiI2(p-methylaniline)4 (Dhital et al., 2020 ▸), again favored by six-coordinate nickel(II) complexes. In the course of our investigations of complexes of substituted aniline ligands, we have encountered one more such compound and here report the synthesis and structure of NiCl2(3-methoxyaniline)4.
The molecule is pseudo-octahedral with trans-chloride ions and all atoms lie on general crystallographic positions (Fig. 1 ▸). The Cl1—Ni1—Cl2 bond angle is nearly linear [179.8 (2)°]. The Cl—Ni—N angles range from 85.45 (5) to 93.82 (5)° while the cis N—Ni—N angles are similar in the range 84.3 (7) to 94.75 (7)° (Table 1 ▸). Taking the NiN4 atoms as the equatorial plane (mean deviation of constituent atoms = 0.0141 Å), the Ni ion lies 0 0029 Å out of the plane. One trans-pair of aniline ligands lie with their C—N bonds oriented nearly in that plane with angles of the C—N vector 2.6 (1)° (C11—N11) or 5.3 (1)° (C21—N21) out of the plane. Conversely, the alternate pair of aniline ligands have their C—N vectors tilted significantly out of the plane at 49.0 (1)° (C31—N31) and 44.0 (1)° (C41—N41). As expected, the aromatic rings are almost planar (mean deviation by ring: N11, 0.0115 Å; N21, 0.0212 Å; N31, 0.0028 Å; N41, 0.0222 Å). The methoxy groups lie very nearly in their respective ring planes as based on the torsion angles [torsion angle Cn7—On3—Cn3—Cn2: n = 1, −10.9 (3)°; 2, −7.8 (3)°; 3, −1.4 (3)°; 4, 179.32 (19)°]. The N41 ring is again unique; the conformations of the methoxy groups of the other three 3-methoxyaniline molecules all show the methoxy group directed toward the amino substituent, while for the N41 ring, it is rotated ∼180° and lies anti to the amino substituent.
Figure 1.
The molecular structure of the title compound with displacement ellipsoids drawn at the 50% probability level. Hydrogen atoms are shown as spheres of arbitrary size. Only those hydrogen atoms whose positions were refined are labeled.
Table 1. Selected geometric parameters (Å, °).
| Ni1—N11 | 2.1388 (19) | Ni1—N41 | 2.2056 (18) |
| Ni1—N21 | 2.1544 (19) | Ni1—Cl1 | 2.3658 (6) |
| Ni1—N31 | 2.1621 (18) | Ni1—Cl2 | 2.4051 (6) |
| N11—Ni1—N21 | 178.52 (8) | N11—Ni1—Cl2 | 89.10 (6) |
| N11—Ni1—N31 | 94.62 (7) | N21—Ni1—Cl2 | 92.06 (6) |
| N21—Ni1—N31 | 86.39 (7) | N31—Ni1—Cl2 | 85.45 (5) |
| N11—Ni1—N41 | 84.25 (7) | N41—Ni1—Cl2 | 93.82 (5) |
| N21—Ni1—N41 | 94.75 (7) | Cl1—Ni1—Cl2 | 179.86 (2) |
| N31—Ni1—N41 | 178.66 (8) | C11—N11—Ni1 | 120.77 (14) |
| N11—Ni1—Cl1 | 90.80 (6) | C21—N21—Ni1 | 116.21 (14) |
| N21—Ni1—Cl1 | 88.04 (6) | C31—N31—Ni1 | 125.09 (14) |
| N31—Ni1—Cl1 | 94.66 (5) | C41—N41—Ni1 | 123.17 (14) |
| N41—Ni1—Cl1 | 86.07 (5) |
It is also noteworthy that the conformations of the anisidine rings are such that three of the rings have their methoxy substituents tipped toward, and above, the Cl2 side of the NiN4 plane. The O33—C33 methoxy group is also tipped in that direction, but due to the orientation of the N31—C31 bond, the methoxy group itself lies on the opposite side of the NiN4 plane.
In the crystal, molecules are linked into chains via weak N—H⋯Cl hydrogen bonds (Table 2 ▸), which results in short contacts between inversion-related chloride ions parallel to the b axis [dCl1⋯Cl1A = 3.725 (2) Å, angleNi1—Cl1⋯Cl1A = 92.4 (1)°; dCl2—Cl2B = 3.721 (2) Å, angleNi1—Cl2⋯Cl2B = 89.3 (1)°; symmetry codes: (A) = 1 − x, 1 − y, 1 − z; (B) = 1 − x, −y, 1 − z] (Fig. 2 ▸). The chains are well separated in both the b- and c-axis directions by the bulk of the 3-methoxyaniline molecules.
Table 2. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N11—H11B⋯Cl1i | 0.83 (2) | 2.44 (3) | 3.264 (2) | 168 (2) |
| N21—H21A⋯Cl2ii | 0.86 (2) | 2.62 (2) | 3.468 (2) | 166 (2) |
| N31—H31B⋯Cl2ii | 0.85 (2) | 2.69 (2) | 3.509 (2) | 160 (2) |
Symmetry codes: (i)  ; (ii)
; (ii)  .
.
Figure 2.
Chain formation via hydrogen bonding (b axis horizontal).
Synthesis and crystallization
Synthesis: 0.5035 g of 3-methoxyaniline were dissolved in 18 ml of EtOH, creating a red solution. NiCl2 hexahydrate was dissolved in 25 ml of EtOH, creating a green solution. Both solutions were heated until they began to boil, at which point the methoxyaniline solution was poured into the nickel chloride solution, resulting in a peach-colored solution that quickly became cloudy. The mixture was repeatedly decanted to remove the majority of the precipitate over the course of two hours and then allowed to cool. The next day, a green powdery precipitate was collected using vacuum filtration and washed using DI water. The filtrate was collected and allowed to evaporate slowly. The next day, small dark-yellow crystals were observed and collected by vacuum filtration, 0.002 g (0.2%).
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 3 ▸.
Table 3. Experimental details.
| Crystal data | |
| Chemical formula | [NiCl2(C7H9NO)4] |
| M r | 622.22 |
| Crystal system, space group | Triclinic, P
|
| Temperature (K) | 100 |
| a, b, c (Å) | 11.4514 (5), 12.1629 (5), 12.6920 (5) |
| α, β, γ (°) | 67.9946 (13), 67.3255 (14), 65.8759 (14) |
| V (Å3) | 1438.34 (11) |
| Z | 2 |
| Radiation type | Mo Kα |
| μ (mm−1) | 0.90 |
| Crystal size (mm) | 0.09 × 0.06 × 0.04 |
| Data collection | |
| Diffractometer | Bruker APEXII CCD |
| Absorption correction | Multi-scan (SADABS; Krause et al., 2015 ▸) |
| Tmin, Tmax | 0.714, 0.746 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 42960, 7138, 4852 |
| R int | 0.076 |
| (sin θ/λ)max (Å−1) | 0.667 |
| Refinement | |
| R[F2 > 2σ(F2)], wR(F2), S | 0.038, 0.089, 1.01 |
| No. of reflections | 7138 |
| No. of parameters | 380 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement |
| Δρmax, Δρmin (e Å−3) | 0.39, −0.32 |
Supplementary Material
Crystal structure: contains datablock(s) I, publication_text. DOI: 10.1107/S2414314624007764/zl4076sup1.cif
CCDC reference: 2376104
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
BAM is grateful for financial support from the Bernard and Vera Kopelman Fund. Author contributions: BAM (synthesis, characterization), DAD (X-ray data), MMT (concept, writing)
full crystallographic data
Dichloridotetrakis(3-methoxyaniline)nickel(II). Crystal data
| [NiCl2(C7H9NO)4] | Z = 2 |
| Mr = 622.22 | F(000) = 652 |
| Triclinic, P1 | Dx = 1.437 Mg m−3 |
| a = 11.4514 (5) Å | Mo Kα radiation, λ = 0.71073 Å |
| b = 12.1629 (5) Å | Cell parameters from 5693 reflections |
| c = 12.6920 (5) Å | θ = 2.9–27.2° |
| α = 67.9946 (13)° | µ = 0.90 mm−1 |
| β = 67.3255 (14)° | T = 100 K |
| γ = 65.8759 (14)° | Plate, yellow |
| V = 1438.34 (11) Å3 | 0.09 × 0.06 × 0.04 mm |
Dichloridotetrakis(3-methoxyaniline)nickel(II). Data collection
| Bruker APEXII CCD diffractometer | 4852 reflections with I > 2σ(I) |
| φ and ω scans | Rint = 0.076 |
| Absorption correction: multi-scan (SADABS; Krause et al., 2015) | θmax = 28.3°, θmin = 2.0° |
| Tmin = 0.714, Tmax = 0.746 | h = −15→15 |
| 42960 measured reflections | k = −16→16 |
| 7138 independent reflections | l = −16→16 |
Dichloridotetrakis(3-methoxyaniline)nickel(II). Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.038 | Hydrogen site location: mixed |
| wR(F2) = 0.089 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.01 | w = 1/[σ2(Fo2) + (0.0334P)2 + 0.4355P] where P = (Fo2 + 2Fc2)/3 |
| 7138 reflections | (Δ/σ)max = 0.001 |
| 380 parameters | Δρmax = 0.39 e Å−3 |
| 0 restraints | Δρmin = −0.32 e Å−3 |
Dichloridotetrakis(3-methoxyaniline)nickel(II). Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Data collection for compound 1 was carried out with a Bruker APEX4 v2022.10–1 CCD diffractometer employing Mo—Kα radiation (λ = 0.71073 Å). The data were collected and reduced using Bruker SMART and SAINT+ software (Bruker, 2014). Absorption corrections were performed using SADABS (Krause, 2015). The structure was solved using SHELXS2014 (Sheldrick, 2008) and refined using SHELXL2018 (Sheldrick, 2015). Hydrogen atoms bonded to carbon atoms were placed geometrically and refined with fixed isotropic thermal parameters, Uiso(H) = 1.2 (C). Hydrogen atoms bonded to nitrogen atoms were located in the difference map and their positions refined with fixed isotropic thermal parameters, Uiso(H) = 1.2 (N) (dN—H = 0.81 (2)–0.91 (2) Å). Final data collection and refinement parameters may be found in Table 2. |
Dichloridotetrakis(3-methoxyaniline)nickel(II). Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Ni1 | 0.50342 (3) | 0.25347 (2) | 0.48264 (2) | 0.01306 (8) | |
| Cl1 | 0.64202 (5) | 0.35611 (5) | 0.47889 (5) | 0.01674 (12) | |
| Cl2 | 0.36209 (5) | 0.14962 (5) | 0.48636 (5) | 0.01653 (12) | |
| N11 | 0.3352 (2) | 0.36558 (18) | 0.58739 (17) | 0.0166 (4) | |
| H11A | 0.275 (2) | 0.378 (2) | 0.558 (2) | 0.020* | |
| H11B | 0.352 (2) | 0.431 (2) | 0.573 (2) | 0.020* | |
| C11 | 0.2928 (2) | 0.31967 (19) | 0.71270 (19) | 0.0157 (4) | |
| C12 | 0.2248 (2) | 0.2323 (2) | 0.75867 (19) | 0.0164 (5) | |
| H12 | 0.205531 | 0.205855 | 0.707310 | 0.020* | |
| O13 | 0.11618 (18) | 0.09999 (16) | 0.93113 (14) | 0.0279 (4) | |
| C13 | 0.1853 (2) | 0.1843 (2) | 0.8798 (2) | 0.0195 (5) | |
| C14 | 0.2155 (2) | 0.2202 (2) | 0.9554 (2) | 0.0222 (5) | |
| H14 | 0.190108 | 0.185492 | 1.038361 | 0.027* | |
| C15 | 0.2830 (2) | 0.3071 (2) | 0.9086 (2) | 0.0223 (5) | |
| H15 | 0.303439 | 0.332402 | 0.960012 | 0.027* | |
| C16 | 0.3215 (2) | 0.3580 (2) | 0.7873 (2) | 0.0185 (5) | |
| H16 | 0.366913 | 0.418434 | 0.755974 | 0.022* | |
| C17 | 0.0651 (3) | 0.0796 (2) | 0.8560 (2) | 0.0306 (6) | |
| H17A | 0.006179 | 0.158736 | 0.820813 | 0.037* | |
| H17B | 0.014986 | 0.020045 | 0.902455 | 0.037* | |
| H17C | 0.139042 | 0.046029 | 0.793178 | 0.037* | |
| N21 | 0.6746 (2) | 0.14469 (17) | 0.37458 (16) | 0.0158 (4) | |
| H21A | 0.664 (2) | 0.072 (2) | 0.397 (2) | 0.019* | |
| H21B | 0.741 (2) | 0.143 (2) | 0.391 (2) | 0.019* | |
| C21 | 0.6950 (2) | 0.1932 (2) | 0.24955 (18) | 0.0162 (5) | |
| O33 | 1.00929 (15) | −0.14771 (14) | 0.62401 (13) | 0.0208 (4) | |
| C22 | 0.6279 (2) | 0.1668 (2) | 0.19530 (19) | 0.0170 (5) | |
| H22 | 0.575639 | 0.112201 | 0.239635 | 0.020* | |
| O23 | 0.57495 (17) | 0.20291 (15) | 0.01482 (13) | 0.0227 (4) | |
| C23 | 0.6384 (2) | 0.2214 (2) | 0.07549 (19) | 0.0185 (5) | |
| C24 | 0.7149 (3) | 0.3015 (2) | 0.0103 (2) | 0.0261 (6) | |
| H24 | 0.721742 | 0.338922 | −0.071467 | 0.031* | |
| C25 | 0.7802 (3) | 0.3257 (2) | 0.0657 (2) | 0.0287 (6) | |
| H25 | 0.832512 | 0.380328 | 0.021309 | 0.034* | |
| C26 | 0.7717 (2) | 0.2719 (2) | 0.1858 (2) | 0.0226 (5) | |
| H26 | 0.817848 | 0.289221 | 0.222808 | 0.027* | |
| C27 | 0.5077 (3) | 0.1107 (2) | 0.0754 (2) | 0.0247 (5) | |
| H27A | 0.569940 | 0.031131 | 0.105624 | 0.030* | |
| H27B | 0.473733 | 0.100612 | 0.020716 | 0.030* | |
| H27C | 0.433351 | 0.137241 | 0.141548 | 0.030* | |
| N31 | 0.55035 (19) | 0.10066 (17) | 0.63226 (16) | 0.0147 (4) | |
| H31A | 0.475 (2) | 0.121 (2) | 0.691 (2) | 0.018* | |
| H31B | 0.551 (2) | 0.040 (2) | 0.614 (2) | 0.018* | |
| C31 | 0.6663 (2) | 0.06435 (19) | 0.67155 (18) | 0.0146 (4) | |
| C32 | 0.7799 (2) | −0.02535 (19) | 0.62731 (18) | 0.0152 (4) | |
| H32 | 0.780233 | −0.061587 | 0.572592 | 0.018* | |
| C33 | 0.8928 (2) | −0.0614 (2) | 0.66390 (19) | 0.0165 (5) | |
| C34 | 0.8918 (2) | −0.0100 (2) | 0.74531 (19) | 0.0200 (5) | |
| H34 | 0.968644 | −0.035787 | 0.771305 | 0.024* | |
| C35 | 0.7780 (2) | 0.0787 (2) | 0.78804 (19) | 0.0205 (5) | |
| H35 | 0.777267 | 0.114153 | 0.843589 | 0.025* | |
| C36 | 0.6646 (2) | 0.1173 (2) | 0.75138 (19) | 0.0187 (5) | |
| H36 | 0.587098 | 0.179170 | 0.780764 | 0.022* | |
| C37 | 1.0107 (2) | −0.2039 (2) | 0.5425 (2) | 0.0223 (5) | |
| H37A | 0.943022 | −0.246875 | 0.579432 | 0.027* | |
| H37B | 1.098606 | −0.264165 | 0.520807 | 0.027* | |
| H37C | 0.991446 | −0.139306 | 0.471444 | 0.027* | |
| N41 | 0.4506 (2) | 0.41029 (18) | 0.33230 (17) | 0.0174 (4) | |
| H41A | 0.464 (2) | 0.465 (2) | 0.343 (2) | 0.021* | |
| H41B | 0.509 (2) | 0.388 (2) | 0.273 (2) | 0.021* | |
| C41 | 0.3218 (2) | 0.4570 (2) | 0.31188 (19) | 0.0158 (5) | |
| C42 | 0.2933 (2) | 0.4009 (2) | 0.25224 (18) | 0.0166 (5) | |
| H42 | 0.360547 | 0.334924 | 0.219965 | 0.020* | |
| O43 | 0.14760 (16) | 0.38059 (15) | 0.17817 (14) | 0.0234 (4) | |
| C43 | 0.1662 (2) | 0.4410 (2) | 0.23955 (19) | 0.0175 (5) | |
| C44 | 0.0670 (2) | 0.5362 (2) | 0.2874 (2) | 0.0232 (5) | |
| H44 | −0.020672 | 0.562393 | 0.280350 | 0.028* | |
| C45 | 0.0980 (3) | 0.5922 (2) | 0.3455 (2) | 0.0285 (6) | |
| H45 | 0.030717 | 0.658162 | 0.377741 | 0.034* | |
| C46 | 0.2242 (2) | 0.5547 (2) | 0.3578 (2) | 0.0221 (5) | |
| H46 | 0.244053 | 0.595015 | 0.397112 | 0.027* | |
| C47 | 0.0172 (2) | 0.4192 (2) | 0.1650 (2) | 0.0251 (5) | |
| H47A | −0.046398 | 0.405454 | 0.243101 | 0.030* | |
| H47B | 0.016330 | 0.370547 | 0.118882 | 0.030* | |
| H47C | −0.007743 | 0.507929 | 0.123847 | 0.030* |
Dichloridotetrakis(3-methoxyaniline)nickel(II). Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Ni1 | 0.01355 (15) | 0.01235 (14) | 0.01499 (15) | −0.00477 (11) | −0.00524 (11) | −0.00311 (10) |
| Cl1 | 0.0156 (3) | 0.0136 (3) | 0.0248 (3) | −0.0050 (2) | −0.0089 (2) | −0.0049 (2) |
| Cl2 | 0.0178 (3) | 0.0147 (3) | 0.0210 (3) | −0.0067 (2) | −0.0081 (2) | −0.0040 (2) |
| N11 | 0.0163 (10) | 0.0139 (9) | 0.0203 (10) | −0.0064 (8) | −0.0050 (8) | −0.0033 (7) |
| C11 | 0.0107 (11) | 0.0146 (11) | 0.0188 (11) | −0.0008 (9) | −0.0029 (9) | −0.0057 (8) |
| C12 | 0.0137 (11) | 0.0171 (11) | 0.0196 (11) | −0.0042 (9) | −0.0056 (9) | −0.0056 (8) |
| O13 | 0.0374 (11) | 0.0331 (10) | 0.0220 (9) | −0.0243 (9) | −0.0084 (8) | −0.0012 (7) |
| C13 | 0.0187 (12) | 0.0155 (11) | 0.0240 (12) | −0.0070 (9) | −0.0049 (10) | −0.0040 (9) |
| C14 | 0.0241 (13) | 0.0240 (12) | 0.0173 (12) | −0.0087 (10) | −0.0059 (10) | −0.0024 (9) |
| C15 | 0.0220 (13) | 0.0238 (12) | 0.0242 (13) | −0.0052 (10) | −0.0089 (10) | −0.0089 (10) |
| C16 | 0.0166 (12) | 0.0169 (11) | 0.0239 (12) | −0.0070 (9) | −0.0058 (10) | −0.0049 (9) |
| C17 | 0.0397 (17) | 0.0384 (15) | 0.0250 (13) | −0.0279 (13) | −0.0076 (12) | −0.0037 (11) |
| N21 | 0.0173 (10) | 0.0148 (10) | 0.0178 (10) | −0.0072 (8) | −0.0061 (8) | −0.0029 (7) |
| C21 | 0.0142 (11) | 0.0167 (11) | 0.0163 (11) | −0.0029 (9) | −0.0023 (9) | −0.0067 (8) |
| O33 | 0.0160 (9) | 0.0248 (9) | 0.0243 (9) | −0.0022 (7) | −0.0067 (7) | −0.0124 (7) |
| C22 | 0.0178 (12) | 0.0150 (11) | 0.0179 (11) | −0.0064 (9) | −0.0029 (9) | −0.0047 (8) |
| O23 | 0.0293 (10) | 0.0268 (9) | 0.0178 (8) | −0.0128 (8) | −0.0097 (7) | −0.0036 (7) |
| C23 | 0.0187 (13) | 0.0201 (12) | 0.0184 (12) | −0.0051 (10) | −0.0046 (10) | −0.0083 (9) |
| C24 | 0.0325 (15) | 0.0320 (14) | 0.0154 (12) | −0.0174 (12) | −0.0047 (10) | −0.0015 (10) |
| C25 | 0.0338 (16) | 0.0341 (15) | 0.0227 (13) | −0.0236 (12) | −0.0036 (11) | −0.0017 (11) |
| C26 | 0.0224 (13) | 0.0291 (13) | 0.0226 (12) | −0.0140 (11) | −0.0073 (10) | −0.0054 (10) |
| C27 | 0.0318 (15) | 0.0255 (13) | 0.0250 (13) | −0.0136 (11) | −0.0137 (11) | −0.0038 (10) |
| N31 | 0.0132 (10) | 0.0129 (9) | 0.0184 (10) | −0.0037 (8) | −0.0042 (8) | −0.0048 (7) |
| C31 | 0.0160 (11) | 0.0138 (11) | 0.0138 (10) | −0.0069 (9) | −0.0052 (9) | 0.0002 (8) |
| C32 | 0.0178 (12) | 0.0150 (11) | 0.0143 (11) | −0.0057 (9) | −0.0047 (9) | −0.0043 (8) |
| C33 | 0.0152 (12) | 0.0156 (11) | 0.0170 (11) | −0.0047 (9) | −0.0037 (9) | −0.0034 (8) |
| C34 | 0.0181 (12) | 0.0254 (12) | 0.0192 (12) | −0.0070 (10) | −0.0080 (9) | −0.0053 (9) |
| C35 | 0.0253 (13) | 0.0244 (12) | 0.0179 (12) | −0.0086 (10) | −0.0073 (10) | −0.0094 (9) |
| C36 | 0.0187 (12) | 0.0180 (11) | 0.0181 (11) | −0.0027 (9) | −0.0047 (9) | −0.0069 (9) |
| C37 | 0.0167 (12) | 0.0255 (13) | 0.0268 (13) | −0.0012 (10) | −0.0053 (10) | −0.0155 (10) |
| N41 | 0.0183 (11) | 0.0167 (10) | 0.0197 (10) | −0.0073 (8) | −0.0075 (8) | −0.0029 (8) |
| C41 | 0.0142 (11) | 0.0150 (11) | 0.0172 (11) | −0.0058 (9) | −0.0063 (9) | 0.0003 (8) |
| C42 | 0.0165 (12) | 0.0162 (11) | 0.0163 (11) | −0.0040 (9) | −0.0045 (9) | −0.0045 (8) |
| O43 | 0.0202 (9) | 0.0271 (9) | 0.0312 (9) | −0.0043 (7) | −0.0129 (7) | −0.0136 (7) |
| C43 | 0.0224 (13) | 0.0173 (11) | 0.0165 (11) | −0.0080 (10) | −0.0092 (9) | −0.0023 (8) |
| C44 | 0.0177 (13) | 0.0260 (13) | 0.0269 (13) | −0.0004 (10) | −0.0112 (10) | −0.0098 (10) |
| C45 | 0.0256 (14) | 0.0267 (13) | 0.0359 (15) | 0.0053 (11) | −0.0149 (12) | −0.0190 (11) |
| C46 | 0.0247 (13) | 0.0210 (12) | 0.0268 (13) | −0.0021 (10) | −0.0153 (11) | −0.0095 (10) |
| C47 | 0.0238 (14) | 0.0313 (14) | 0.0282 (13) | −0.0106 (11) | −0.0132 (11) | −0.0074 (10) |
Dichloridotetrakis(3-methoxyaniline)nickel(II). Geometric parameters (Å, º)
| Ni1—N11 | 2.1388 (19) | C25—H25 | 0.9500 |
| Ni1—N21 | 2.1544 (19) | C26—H26 | 0.9500 |
| Ni1—N31 | 2.1621 (18) | C27—H27A | 0.9800 |
| Ni1—N41 | 2.2056 (18) | C27—H27B | 0.9800 |
| Ni1—Cl1 | 2.3658 (6) | C27—H27C | 0.9800 |
| Ni1—Cl2 | 2.4051 (6) | N31—C31 | 1.440 (3) |
| N11—C11 | 1.428 (3) | N31—H31A | 0.91 (2) |
| N11—H11A | 0.85 (2) | N31—H31B | 0.85 (2) |
| N11—H11B | 0.83 (2) | C31—C36 | 1.381 (3) |
| C11—C16 | 1.385 (3) | C31—C32 | 1.391 (3) |
| C11—C12 | 1.392 (3) | C32—C33 | 1.388 (3) |
| C12—C13 | 1.384 (3) | C32—H32 | 0.9500 |
| C12—H12 | 0.9500 | C33—C34 | 1.388 (3) |
| O13—C13 | 1.367 (3) | C34—C35 | 1.379 (3) |
| O13—C17 | 1.428 (3) | C34—H34 | 0.9500 |
| C13—C14 | 1.387 (3) | C35—C36 | 1.388 (3) |
| C14—C15 | 1.382 (3) | C35—H35 | 0.9500 |
| C14—H14 | 0.9500 | C36—H36 | 0.9500 |
| C15—C16 | 1.390 (3) | C37—H37A | 0.9800 |
| C15—H15 | 0.9500 | C37—H37B | 0.9800 |
| C16—H16 | 0.9500 | C37—H37C | 0.9800 |
| C17—H17A | 0.9800 | N41—C41 | 1.436 (3) |
| C17—H17B | 0.9800 | N41—H41A | 0.81 (2) |
| C17—H17C | 0.9800 | N41—H41B | 0.83 (2) |
| N21—C21 | 1.430 (3) | C41—C42 | 1.381 (3) |
| N21—H21A | 0.86 (2) | C41—C46 | 1.390 (3) |
| N21—H21B | 0.85 (2) | C42—C43 | 1.387 (3) |
| C21—C26 | 1.379 (3) | C42—H42 | 0.9500 |
| C21—C22 | 1.393 (3) | O43—C43 | 1.370 (3) |
| O33—C33 | 1.368 (3) | O43—C47 | 1.428 (3) |
| O33—C37 | 1.431 (3) | C43—C44 | 1.385 (3) |
| C22—C23 | 1.391 (3) | C44—C45 | 1.383 (3) |
| C22—H22 | 0.9500 | C44—H44 | 0.9500 |
| O23—C23 | 1.366 (3) | C45—C46 | 1.380 (3) |
| O23—C27 | 1.430 (3) | C45—H45 | 0.9500 |
| C23—C24 | 1.391 (3) | C46—H46 | 0.9500 |
| C24—C25 | 1.371 (3) | C47—H47A | 0.9800 |
| C24—H24 | 0.9500 | C47—H47B | 0.9800 |
| C25—C26 | 1.397 (3) | C47—H47C | 0.9800 |
| N11—Ni1—N21 | 178.52 (8) | C21—C26—H26 | 120.6 |
| N11—Ni1—N31 | 94.62 (7) | C25—C26—H26 | 120.6 |
| N21—Ni1—N31 | 86.39 (7) | O23—C27—H27A | 109.5 |
| N11—Ni1—N41 | 84.25 (7) | O23—C27—H27B | 109.5 |
| N21—Ni1—N41 | 94.75 (7) | H27A—C27—H27B | 109.5 |
| N31—Ni1—N41 | 178.66 (8) | O23—C27—H27C | 109.5 |
| N11—Ni1—Cl1 | 90.80 (6) | H27A—C27—H27C | 109.5 |
| N21—Ni1—Cl1 | 88.04 (6) | H27B—C27—H27C | 109.5 |
| N31—Ni1—Cl1 | 94.66 (5) | C31—N31—Ni1 | 125.09 (14) |
| N41—Ni1—Cl1 | 86.07 (5) | C31—N31—H31A | 110.5 (15) |
| N11—Ni1—Cl2 | 89.10 (6) | Ni1—N31—H31A | 100.9 (14) |
| N21—Ni1—Cl2 | 92.06 (6) | C31—N31—H31B | 109.2 (16) |
| N31—Ni1—Cl2 | 85.45 (5) | Ni1—N31—H31B | 101.4 (16) |
| N41—Ni1—Cl2 | 93.82 (5) | H31A—N31—H31B | 109 (2) |
| Cl1—Ni1—Cl2 | 179.86 (2) | C36—C31—C32 | 120.8 (2) |
| C11—N11—Ni1 | 120.77 (14) | C36—C31—N31 | 120.7 (2) |
| C11—N11—H11A | 109.6 (16) | C32—C31—N31 | 118.46 (19) |
| Ni1—N11—H11A | 100.8 (16) | C33—C32—C31 | 119.3 (2) |
| C11—N11—H11B | 107.7 (16) | C33—C32—H32 | 120.3 |
| Ni1—N11—H11B | 106.1 (17) | C31—C32—H32 | 120.3 |
| H11A—N11—H11B | 112 (2) | O33—C33—C32 | 123.3 (2) |
| C16—C11—C12 | 120.4 (2) | O33—C33—C34 | 116.24 (19) |
| C16—C11—N11 | 121.1 (2) | C32—C33—C34 | 120.4 (2) |
| C12—C11—N11 | 118.4 (2) | C35—C34—C33 | 119.3 (2) |
| C13—C12—C11 | 119.5 (2) | C35—C34—H34 | 120.4 |
| C13—C12—H12 | 120.3 | C33—C34—H34 | 120.4 |
| C11—C12—H12 | 120.3 | C34—C35—C36 | 121.2 (2) |
| C13—O13—C17 | 116.54 (18) | C34—C35—H35 | 119.4 |
| O13—C13—C12 | 122.6 (2) | C36—C35—H35 | 119.4 |
| O13—C13—C14 | 116.7 (2) | C31—C36—C35 | 119.0 (2) |
| C12—C13—C14 | 120.7 (2) | C31—C36—H36 | 120.5 |
| C15—C14—C13 | 119.2 (2) | C35—C36—H36 | 120.5 |
| C15—C14—H14 | 120.4 | O33—C37—H37A | 109.5 |
| C13—C14—H14 | 120.4 | O33—C37—H37B | 109.5 |
| C14—C15—C16 | 121.0 (2) | H37A—C37—H37B | 109.5 |
| C14—C15—H15 | 119.5 | O33—C37—H37C | 109.5 |
| C16—C15—H15 | 119.5 | H37A—C37—H37C | 109.5 |
| C11—C16—C15 | 119.2 (2) | H37B—C37—H37C | 109.5 |
| C11—C16—H16 | 120.4 | C41—N41—Ni1 | 123.17 (14) |
| C15—C16—H16 | 120.4 | C41—N41—H41A | 109.1 (18) |
| O13—C17—H17A | 109.5 | Ni1—N41—H41A | 101.2 (17) |
| O13—C17—H17B | 109.5 | C41—N41—H41B | 109.4 (17) |
| H17A—C17—H17B | 109.5 | Ni1—N41—H41B | 104.0 (17) |
| O13—C17—H17C | 109.5 | H41A—N41—H41B | 109 (2) |
| H17A—C17—H17C | 109.5 | C42—C41—C46 | 120.3 (2) |
| H17B—C17—H17C | 109.5 | C42—C41—N41 | 120.1 (2) |
| C21—N21—Ni1 | 116.21 (14) | C46—C41—N41 | 119.6 (2) |
| C21—N21—H21A | 109.8 (15) | C41—C42—C43 | 119.9 (2) |
| Ni1—N21—H21A | 105.2 (16) | C41—C42—H42 | 120.1 |
| C21—N21—H21B | 107.8 (16) | C43—C42—H42 | 120.1 |
| Ni1—N21—H21B | 104.7 (16) | C43—O43—C47 | 116.84 (18) |
| H21A—N21—H21B | 113 (2) | O43—C43—C44 | 123.7 (2) |
| C26—C21—C22 | 120.7 (2) | O43—C43—C42 | 115.7 (2) |
| C26—C21—N21 | 120.4 (2) | C44—C43—C42 | 120.6 (2) |
| C22—C21—N21 | 118.6 (2) | C45—C44—C43 | 118.7 (2) |
| C33—O33—C37 | 116.88 (17) | C45—C44—H44 | 120.7 |
| C23—C22—C21 | 119.3 (2) | C43—C44—H44 | 120.7 |
| C23—C22—H22 | 120.4 | C46—C45—C44 | 121.7 (2) |
| C21—C22—H22 | 120.4 | C46—C45—H45 | 119.2 |
| C23—O23—C27 | 117.27 (17) | C44—C45—H45 | 119.2 |
| O23—C23—C24 | 115.8 (2) | C45—C46—C41 | 119.0 (2) |
| O23—C23—C22 | 123.7 (2) | C45—C46—H46 | 120.5 |
| C24—C23—C22 | 120.5 (2) | C41—C46—H46 | 120.5 |
| C25—C24—C23 | 119.1 (2) | O43—C47—H47A | 109.5 |
| C25—C24—H24 | 120.5 | O43—C47—H47B | 109.5 |
| C23—C24—H24 | 120.5 | H47A—C47—H47B | 109.5 |
| C24—C25—C26 | 121.6 (2) | O43—C47—H47C | 109.5 |
| C24—C25—H25 | 119.2 | H47A—C47—H47C | 109.5 |
| C26—C25—H25 | 119.2 | H47B—C47—H47C | 109.5 |
| C21—C26—C25 | 118.8 (2) | ||
| Ni1—N11—C11—C16 | 102.6 (2) | Ni1—N31—C31—C36 | 88.5 (2) |
| Ni1—N11—C11—C12 | −75.6 (2) | Ni1—N31—C31—C32 | −91.6 (2) |
| C16—C11—C12—C13 | 0.3 (3) | C36—C31—C32—C33 | −0.2 (3) |
| N11—C11—C12—C13 | 178.5 (2) | N31—C31—C32—C33 | 179.85 (19) |
| C17—O13—C13—C12 | −10.9 (3) | C37—O33—C33—C32 | −1.4 (3) |
| C17—O13—C13—C14 | 169.4 (2) | C37—O33—C33—C34 | 178.35 (19) |
| C11—C12—C13—O13 | 178.8 (2) | C31—C32—C33—O33 | −179.19 (19) |
| C11—C12—C13—C14 | −1.5 (3) | C31—C32—C33—C34 | 1.1 (3) |
| O13—C13—C14—C15 | −178.7 (2) | O33—C33—C34—C35 | 179.2 (2) |
| C12—C13—C14—C15 | 1.5 (4) | C32—C33—C34—C35 | −1.0 (3) |
| C13—C14—C15—C16 | −0.4 (4) | C33—C34—C35—C36 | 0.2 (3) |
| C12—C11—C16—C15 | 0.8 (3) | C32—C31—C36—C35 | −0.6 (3) |
| N11—C11—C16—C15 | −177.4 (2) | N31—C31—C36—C35 | 179.3 (2) |
| C14—C15—C16—C11 | −0.8 (4) | C34—C35—C36—C31 | 0.7 (3) |
| Ni1—N21—C21—C26 | 90.7 (2) | Ni1—N41—C41—C42 | −84.4 (2) |
| Ni1—N21—C21—C22 | −84.6 (2) | Ni1—N41—C41—C46 | 92.3 (2) |
| C26—C21—C22—C23 | −0.3 (3) | C46—C41—C42—C43 | −0.9 (3) |
| N21—C21—C22—C23 | 174.9 (2) | N41—C41—C42—C43 | 175.75 (19) |
| C27—O23—C23—C24 | 173.2 (2) | C47—O43—C43—C44 | −0.5 (3) |
| C27—O23—C23—C22 | −7.8 (3) | C47—O43—C43—C42 | 179.32 (19) |
| C21—C22—C23—O23 | −179.01 (19) | C41—C42—C43—O43 | 179.42 (19) |
| C21—C22—C23—C24 | 0.0 (3) | C41—C42—C43—C44 | −0.7 (3) |
| O23—C23—C24—C25 | 179.2 (2) | O43—C43—C44—C45 | −178.6 (2) |
| C22—C23—C24—C25 | 0.2 (4) | C42—C43—C44—C45 | 1.5 (3) |
| C23—C24—C25—C26 | 0.0 (4) | C43—C44—C45—C46 | −0.7 (4) |
| C22—C21—C26—C25 | 0.5 (3) | C44—C45—C46—C41 | −0.9 (4) |
| N21—C21—C26—C25 | −174.7 (2) | C42—C41—C46—C45 | 1.7 (3) |
| C24—C25—C26—C21 | −0.3 (4) | N41—C41—C46—C45 | −175.0 (2) |
Dichloridotetrakis(3-methoxyaniline)nickel(II). Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N11—H11B···Cl1i | 0.83 (2) | 2.44 (3) | 3.264 (2) | 168 (2) |
| N21—H21A···Cl2ii | 0.86 (2) | 2.62 (2) | 3.468 (2) | 166 (2) |
| N31—H31B···Cl2ii | 0.85 (2) | 2.69 (2) | 3.509 (2) | 160 (2) |
Symmetry codes: (i) −x+1, −y+1, −z+1; (ii) −x+1, −y, −z+1.
References
- Amani, V. (2018). J. Mol. Struct.1155, 477–483.
- Bruker (2022). APEX4 and SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
- Burrow, R. A., Hörner, M., Lang, L. S., Neves, A. & Vencato, I. (1997). Z. Kristallogr. New Cryst. Struct.212, 41–42.
- Chellali, J. E., Keely, C., Bell, G., Dimanno, K. L., Tran, T., Landee, C. P., Dickie, D. A., Rademeyer, M., Turnbull, M. M. & Xiao, F. (2019). Polyhedron, 168, 1–10.
- Chen, Y.-B., Li, Z.-J., Qin, Y.-Y., Kang, Y., Wu, L. & Yao, Y.-G. (2002). Jiegou Huaxue, 21, 530–2.
- Clegg, W. & Martin, N. C. (2007). Acta Cryst. E63, m856.
- Costin-Hogan, C. E., Chen, C.-L., Hughes, E., Pickett, A., Valencia, R., Rath, N. P. & Beatty, A. M. (2008). CrystEngComm, 10, 1910–1915.
- Daniliuc, C. G., Kotov, V., Frohlich, R. & Erker, G. (2023). CSD Communication (CCDC 2308702). CCDC, Cambridge, England.
- Dhital, R. N., Sen, A., Sato, T., Hu, H., Ishii, R., Hashizume, D., Takaya, H., Uozumi, Y. & Yamada, Y. M. A. (2020). Org. Lett.22, 4797–4801. [DOI] [PubMed]
- Ejaz, Sahin, O. & Khan, I. U. (2009). Acta Cryst. E65, m1457. [DOI] [PMC free article] [PubMed]
- Fawcett, J., Sicilia, F. & Solan, G. A. (2005). Acta Cryst. E61, m1256–m1257.
- Govindaraj, J., Thirumurugan, S., Reddy, D. S., Anbalagan, K. & SubbiahPandi, A. (2015). Acta Cryst. E71, m21–m22. [DOI] [PMC free article] [PubMed]
- Guedes, G. P., Farias, F. F., Novak, M. A., Machado, A. & Vaz, F. L. (2011). Inorg. Chim. Acta, 378, 134–139.
- Harmouzi, A., Daro, N., Guionneau, P., Belaaraj, A. & Khechoubi, E. M. (2017). J. Cryst. Growth, 472, 64–70.
- Khan, I. U., Ejaz, Şahin, O. & Büyükgüngör, O. (2010). Acta Cryst. E66, m492. [DOI] [PMC free article] [PubMed]
- Krause, L., Herbst-Irmer, R., Sheldrick, G. M. & Stalke, D. (2015). J. Appl. Cryst.48, 3–10. [DOI] [PMC free article] [PubMed]
- Kupko, N., Meehan, K. L., Witkos, F. E., Hutcheson, H., Monroe, J. C., Landee, C. P., Dickie, D. A., Turnbull, M. M. & Xiao, F. (2020). Polyhedron, 187, 1146801-13.
- Li, X. (2023). CSD Communication (CCDC 2223797). CCDC, Cambridge, England.
- Löw, S., Becker, J., Würtele, C., Miska, A., Kleeberg, C., Behrens, U., Walter, O. & Schindler, S. (2013). Chem. A Eur. J.19, 5342–5351. [DOI] [PubMed]
- Macek, L., Bellamy, J. C., Faber, K., Milson, C. R., Landee, C. P., Dickie, D. A. & Turnbull, M. M. (2023). Polyhedron, 229, 1162141-1162145.
- Meehan, K. L., Fontaine, D. F. A., Richardson, A. D., Fowles, S. M., Mukda, B., Monroe, J. C., Landee, C. P., Dickie, D. A., Turnbull, M. M., Jiang, S. & Xiao, F. (2021). Polyhedron, 200, 1150941.
- Nemec, V., Lisac, K., Liovic, M., Brekalo, I. & Cincic, D. (2020). Materials13, 2385. [DOI] [PMC free article] [PubMed]
- Rademeyer, M. (2004). Acta Cryst. E60, m871–m872. [DOI] [PubMed]
- Rademeyer, M., Overbeek, G. E. & Liles, D. C. (2010). Acta Cryst. E66, m1634. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Sheldrick, G. M. (2015). Acta Cryst. C71, 3–8.
- Subashini, A., Ramamurthi, K. & Stoeckli-Evans, H. (2012a). Acta Cryst. E68, m1152. [DOI] [PMC free article] [PubMed]
- Subashini, A., Ramamurthi, K. & Stoeckli-Evans, H. (2012b). CSD Communication (CCDC 894045). CCDC, Cambridge, England.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, publication_text. DOI: 10.1107/S2414314624007764/zl4076sup1.cif
CCDC reference: 2376104
Additional supporting information: crystallographic information; 3D view; checkCIF report




