Abstract
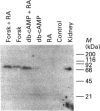
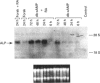
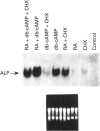
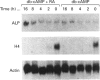
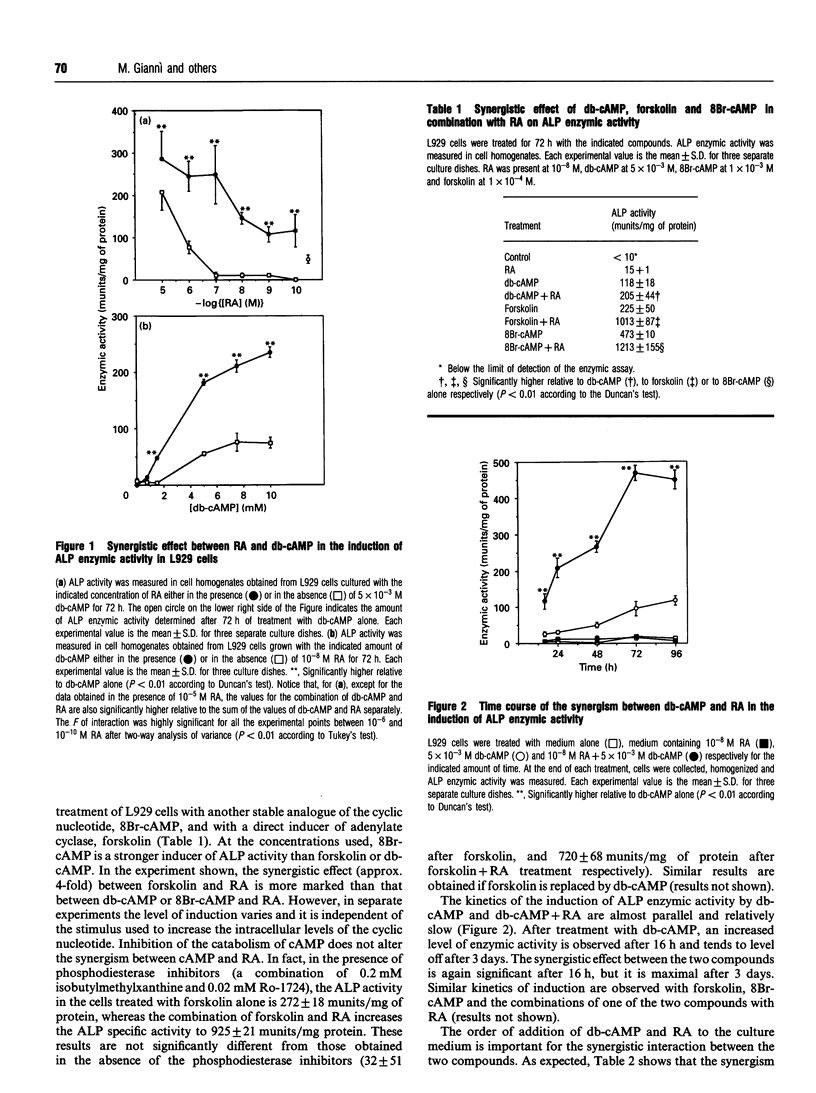
In L929 mouse fibroblastic cells, liver/bone/kidney type alkaline phosphatase (L/B/K-ALP) enzymic activity is induced by all-trans-retinoic acid at concentrations between 10(-6) and 10(-5) M. At lower concentrations, retinoic acid is incapable of inducing this enzymic activity per se, but increases cyclic AMP (cAMP)-mediated induction. This effect is observed after incubation of the retinoid with dibutyryl cAMP, 8-bromo cAMP or forskolin. The synergism is dependent on the order of addition of retinoic acid and the activator of the cAMP pathway. Contemporaneous addition of the two agents, or addition of cAMP prior to retinoic acid (but not addition of retinoic acid before cAMP), is necessary to produce this synergistic interaction. The synergism results in increased steady-state levels of L/B/K-ALP mRNA and it is the consequence of increased transcriptional activity of the gene. The expression of the mouse L/B/K-ALP gene is regulated by the presence of two leader exons, 1A and 1B, resulting in the synthesis of two alternatively spliced mRNAs that are different only in part of their 5' untranslated region [Studer, Terao, Giannì and Garattini (1991) Biochem. Biophys. Res. Commun. 179, 1352-1360]. PCR amplification and nuclear run-on experiments performed using probes specific for each leader exon demonstrate that treatment of these cells with retinoic acid, forskolin or dibutyryl cAMP, and with the combination of the retinoid and one of the cAMP-elevating agents, leads to the accumulation of nascent and mature L/B/K-ALP mRNA containing exon 1B. The synergistic induction of the transcription of the L/B/K-ALP gene is well correlated with quantitative and qualitative changes of retinoic-acid-receptor mRNAs mediated by cAMP.
Full text
PDF


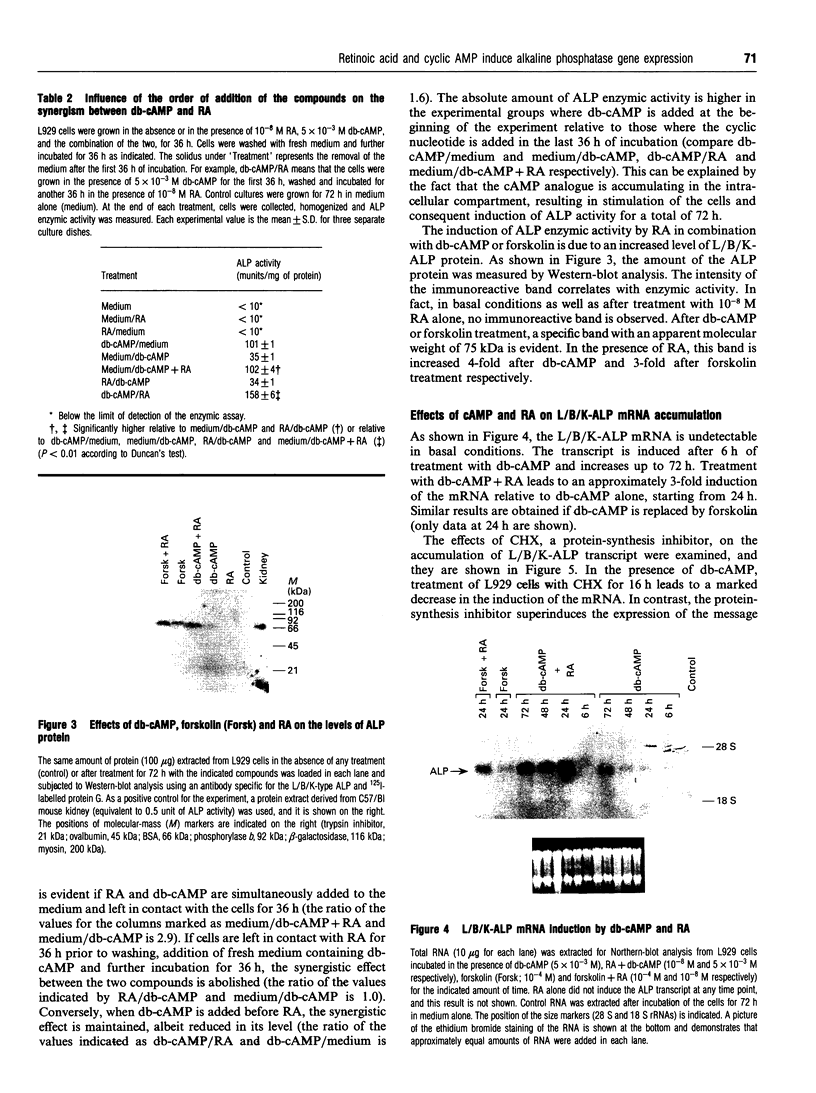


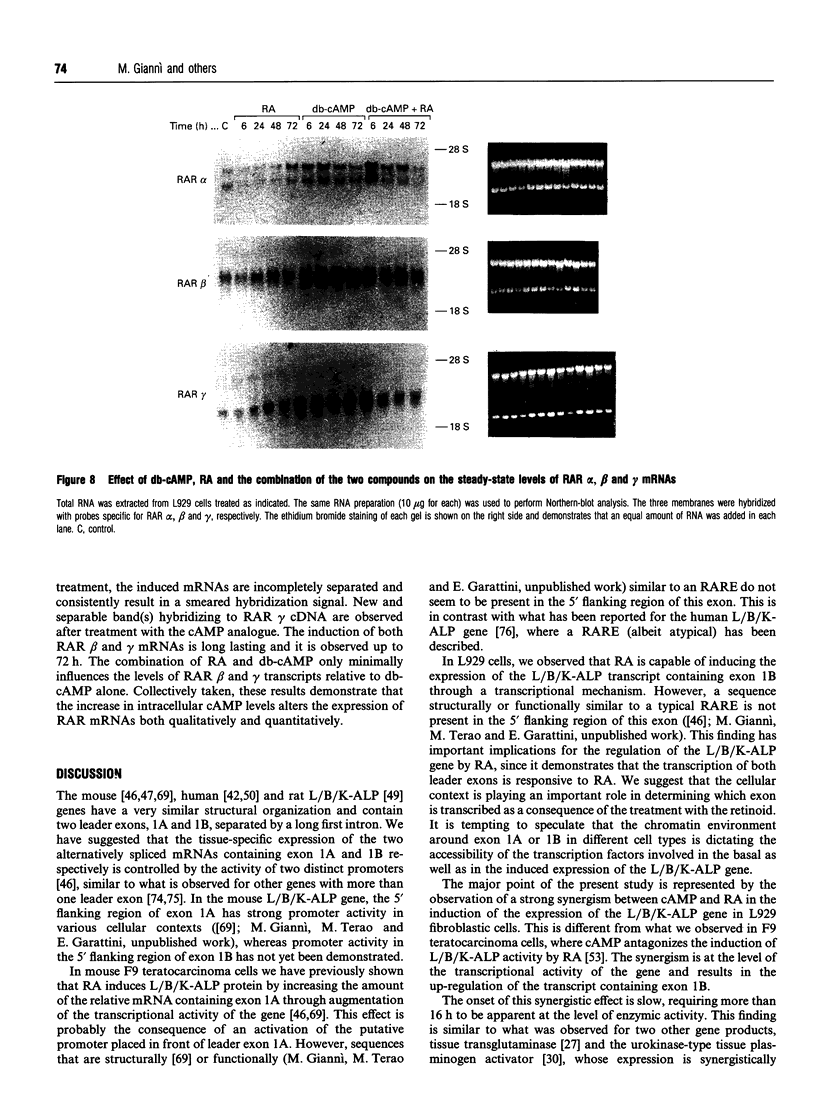
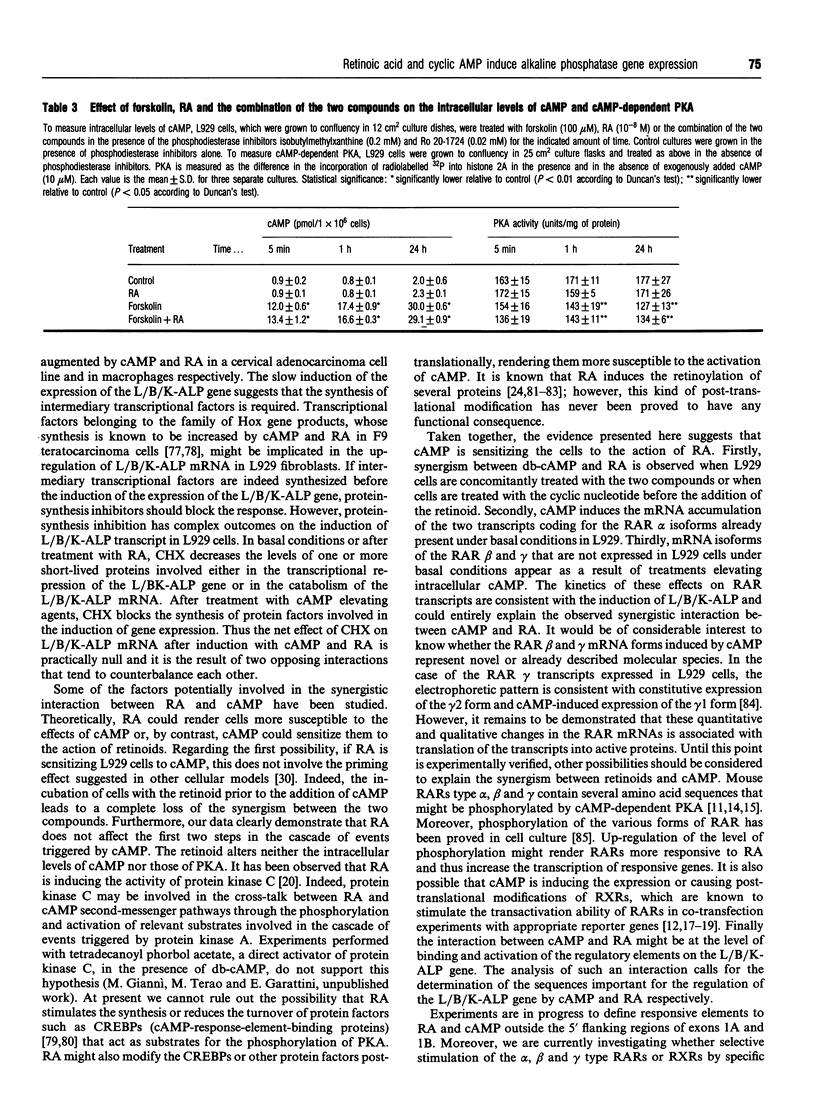


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso S., Minty A., Bourlet Y., Buckingham M. Comparison of three actin-coding sequences in the mouse; evolutionary relationships between the actin genes of warm-blooded vertebrates. J Mol Evol. 1986;23(1):11–22. doi: 10.1007/BF02100994. [DOI] [PubMed] [Google Scholar]
- Bailey J. S., Siu C. H. Purification and partial characterization of a novel binding protein for retinoic acid from neonatal rat. J Biol Chem. 1988 Jul 5;263(19):9326–9332. [PubMed] [Google Scholar]
- Berger J., Garattini E., Hua J. C., Udenfriend S. Cloning and sequencing of human intestinal alkaline phosphatase cDNA. Proc Natl Acad Sci U S A. 1987 Feb;84(3):695–698. doi: 10.1073/pnas.84.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard B. A., Bernardon J. M., Delescluse C., Martin B., Lenoir M. C., Maignan J., Charpentier B., Pilgrim W. R., Reichert U., Shroot B. Identification of synthetic retinoids with selectivity for human nuclear retinoic acid receptor gamma. Biochem Biophys Res Commun. 1992 Jul 31;186(2):977–983. doi: 10.1016/0006-291x(92)90842-9. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Breier G., Bućan M., Francke U., Colberg-Poley A. M., Gruss P. Sequential expression of murine homeo box genes during F9 EC cell differentiation. EMBO J. 1986 Sep;5(9):2209–2215. doi: 10.1002/j.1460-2075.1986.tb04486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitman T. R., Selonick S. E., Collins S. J. Induction of differentiation of the human promyelocytic leukemia cell line (HL-60) by retinoic acid. Proc Natl Acad Sci U S A. 1980 May;77(5):2936–2940. doi: 10.1073/pnas.77.5.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N. A., Stofko R. E., Uhler M. D. Induction of alkaline phosphatase in mouse L cells by overexpression of the catalytic subunit of cAMP-dependent protein kinase. J Biol Chem. 1990 Aug 5;265(22):13181–13189. [PubMed] [Google Scholar]
- Bugge T. H., Pohl J., Lonnoy O., Stunnenberg H. G. RXR alpha, a promiscuous partner of retinoic acid and thyroid hormone receptors. EMBO J. 1992 Apr;11(4):1409–1418. doi: 10.1002/j.1460-2075.1992.tb05186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colberg-Poley A. M., Voss S. D., Chowdhury K., Gruss P. Structural analysis of murine genes containing homoeo box sequences and their expression in embryonal carcinoma cells. 1985 Apr 25-May 1Nature. 314(6013):713–718. doi: 10.1038/314713a0. [DOI] [PubMed] [Google Scholar]
- Delescluse C., Cavey M. T., Martin B., Bernard B. A., Reichert U., Maignan J., Darmon M., Shroot B. Selective high affinity retinoic acid receptor alpha or beta-gamma ligands. Mol Pharmacol. 1991 Oct;40(4):556–562. [PubMed] [Google Scholar]
- Eick D., Bornkamm G. W. Transcriptional arrest within the first exon is a fast control mechanism in c-myc gene expression. Nucleic Acids Res. 1986 Nov 11;14(21):8331–8346. doi: 10.1093/nar/14.21.8331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Firestone G. L., Heath E. C. The cyclic AMP-mediated induction of alkaline phosphatase in mouse L-cells. J Biol Chem. 1981 Feb 10;256(3):1396–1403. [PubMed] [Google Scholar]
- Garattini E., Hua J. C., Pan Y. C., Udenfriend S. Human liver alkaline phosphatase, purification and partial sequencing: homology with the placental isozyme. Arch Biochem Biophys. 1986 Mar;245(2):331–337. doi: 10.1016/0003-9861(86)90223-7. [DOI] [PubMed] [Google Scholar]
- Garattini E., Hua J. C., Udenfriend S. Cloning and sequencing of bovine kidney alkaline phosphatase cDNA. Gene. 1987;59(1):41–46. doi: 10.1016/0378-1119(87)90264-2. [DOI] [PubMed] [Google Scholar]
- Gaub M. P., Rochette-Egly C., Lutz Y., Ali S., Matthes H., Scheuer I., Chambon P. Immunodetection of multiple species of retinoic acid receptor alpha: evidence for phosphorylation. Exp Cell Res. 1992 Aug;201(2):335–346. doi: 10.1016/0014-4827(92)90282-d. [DOI] [PubMed] [Google Scholar]
- Gianni M., Studer M., Carpani G., Terao M., Garattini E. Retinoic acid induces liver/bone/kidney-type alkaline phosphatase gene expression in F9 teratocarcinoma cells. Biochem J. 1991 Mar 15;274(Pt 3):673–678. doi: 10.1042/bj2740673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giguere V., Ong E. S., Segui P., Evans R. M. Identification of a receptor for the morphogen retinoic acid. Nature. 1987 Dec 17;330(6149):624–629. doi: 10.1038/330624a0. [DOI] [PubMed] [Google Scholar]
- Giguère V., Lyn S., Yip P., Siu C. H., Amin S. Molecular cloning of cDNA encoding a second cellular retinoic acid-binding protein. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6233–6237. doi: 10.1073/pnas.87.16.6233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez G. A., Yamamoto K. K., Fischer W. H., Karr D., Menzel P., Biggs W., 3rd, Vale W. W., Montminy M. R. A cluster of phosphorylation sites on the cyclic AMP-regulated nuclear factor CREB predicted by its sequence. Nature. 1989 Feb 23;337(6209):749–752. doi: 10.1038/337749a0. [DOI] [PubMed] [Google Scholar]
- Green E., Todd B., Heath D. Mechanism of glucocorticoid regulation of alkaline phosphatase gene expression in osteoblast-like cells. Eur J Biochem. 1990 Feb 22;188(1):147–153. doi: 10.1111/j.1432-1033.1990.tb15382.x. [DOI] [PubMed] [Google Scholar]
- Gruber J. R., Ohno S., Niles R. M. Increased expression of protein kinase C alpha plays a key role in retinoic acid-induced melanoma differentiation. J Biol Chem. 1992 Jul 5;267(19):13356–13360. [PubMed] [Google Scholar]
- Hagenbüchle O., Tosi M., Schibler U., Bovey R., Wellauer P. K., Young R. A. Mouse liver and salivary gland alpha-amylase mRNAs differ only in 5' non-translated sequences. Nature. 1981 Feb 19;289(5799):643–646. doi: 10.1038/289643a0. [DOI] [PubMed] [Google Scholar]
- Heath J. K., Suva L. J., Yoon K., Kiledjian M., Martin T. J., Rodan G. A. Retinoic acid stimulates transcriptional activity from the alkaline phosphatase promoter in the immortalized rat calvarial cell line, RCT-1. Mol Endocrinol. 1992 Apr;6(4):636–646. doi: 10.1210/mend.6.4.1584226. [DOI] [PubMed] [Google Scholar]
- Henthorn P. S., Raducha M., Edwards Y. H., Weiss M. J., Slaughter C., Lafferty M. A., Harris H. Nucleotide and amino acid sequences of human intestinal alkaline phosphatase: close homology to placental alkaline phosphatase. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1234–1238. doi: 10.1073/pnas.84.5.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyman R. A., Mangelsdorf D. J., Dyck J. A., Stein R. B., Eichele G., Evans R. M., Thaller C. 9-cis retinoic acid is a high affinity ligand for the retinoid X receptor. Cell. 1992 Jan 24;68(2):397–406. doi: 10.1016/0092-8674(92)90479-v. [DOI] [PubMed] [Google Scholar]
- Hoeffler J. P., Meyer T. E., Yun Y., Jameson J. L., Habener J. F. Cyclic AMP-responsive DNA-binding protein: structure based on a cloned placental cDNA. Science. 1988 Dec 9;242(4884):1430–1433. doi: 10.1126/science.2974179. [DOI] [PubMed] [Google Scholar]
- Hu L., Gudas L. J. Cyclic AMP analogs and retinoic acid influence the expression of retinoic acid receptor alpha, beta, and gamma mRNAs in F9 teratocarcinoma cells. Mol Cell Biol. 1990 Jan;10(1):391–396. doi: 10.1128/mcb.10.1.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner P., Krust A., Mendelsohn C., Garnier J. M., Zelent A., Leroy P., Staub A., Chambon P. Murine isoforms of retinoic acid receptor gamma with specific patterns of expression. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2700–2704. doi: 10.1073/pnas.87.7.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamoto T., Momoi T., Momoi M. The presence of a novel cellular retinoic acid-binding protein in chick embryos: purification and partial characterization. Biochem Biophys Res Commun. 1988 Dec 30;157(3):1302–1308. doi: 10.1016/s0006-291x(88)81016-7. [DOI] [PubMed] [Google Scholar]
- Kliewer S. A., Umesono K., Mangelsdorf D. J., Evans R. M. Retinoid X receptor interacts with nuclear receptors in retinoic acid, thyroid hormone and vitamin D3 signalling. Nature. 1992 Jan 30;355(6359):446–449. doi: 10.1038/355446a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll B. J., Rothblum K. N., Longley M. Nucleotide sequence of the human placental alkaline phosphatase gene. Evolution of the 5' flanking region by deletion/substitution. J Biol Chem. 1988 Aug 25;263(24):12020–12027. [PubMed] [Google Scholar]
- Krust A., Kastner P., Petkovich M., Zelent A., Chambon P. A third human retinoic acid receptor, hRAR-gamma. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5310–5314. doi: 10.1073/pnas.86.14.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRosa G. J., Gudas L. J. An early effect of retinoic acid: cloning of an mRNA (Era-1) exhibiting rapid and protein synthesis-independent induction during teratocarcinoma stem cell differentiation. Proc Natl Acad Sci U S A. 1988 Jan;85(2):329–333. doi: 10.1073/pnas.85.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leid M., Kastner P., Lyons R., Nakshatri H., Saunders M., Zacharewski T., Chen J. Y., Staub A., Garnier J. M., Mader S. Purification, cloning, and RXR identity of the HeLa cell factor with which RAR or TR heterodimerizes to bind target sequences efficiently. Cell. 1992 Jan 24;68(2):377–395. doi: 10.1016/0092-8674(92)90478-u. [DOI] [PubMed] [Google Scholar]
- Leroy P., Krust A., Zelent A., Mendelsohn C., Garnier J. M., Kastner P., Dierich A., Chambon P. Multiple isoforms of the mouse retinoic acid receptor alpha are generated by alternative splicing and differential induction by retinoic acid. EMBO J. 1991 Jan;10(1):59–69. doi: 10.1002/j.1460-2075.1991.tb07921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin A. A., Sturzenbecker L. J., Kazmer S., Bosakowski T., Huselton C., Allenby G., Speck J., Kratzeisen C., Rosenberger M., Lovey A. 9-cis retinoic acid stereoisomer binds and activates the nuclear receptor RXR alpha. Nature. 1992 Jan 23;355(6358):359–361. doi: 10.1038/355359a0. [DOI] [PubMed] [Google Scholar]
- Lotan R. Effects of vitamin A and its analogs (retinoids) on normal and neoplastic cells. Biochim Biophys Acta. 1980 Mar 12;605(1):33–91. doi: 10.1016/0304-419x(80)90021-9. [DOI] [PubMed] [Google Scholar]
- Makiya R., Stigbrand T. Placental alkaline phosphatase has a binding site for the human immunoglobulin-G Fc portion. Eur J Biochem. 1992 Apr 1;205(1):341–345. doi: 10.1111/j.1432-1033.1992.tb16785.x. [DOI] [PubMed] [Google Scholar]
- Manes T., Glade K., Ziomek C. A., Millán J. L. Genomic structure and comparison of mouse tissue-specific alkaline phosphatase genes. Genomics. 1990 Nov;8(3):541–554. doi: 10.1016/0888-7543(90)90042-s. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D. J., Umesono K., Kliewer S. A., Borgmeyer U., Ong E. S., Evans R. M. A direct repeat in the cellular retinol-binding protein type II gene confers differential regulation by RXR and RAR. Cell. 1991 Aug 9;66(3):555–561. doi: 10.1016/0092-8674(81)90018-0. [DOI] [PubMed] [Google Scholar]
- Matsuura S., Kishi F., Kajii T. Characterization of a 5'-flanking region of the human liver/bone/kidney alkaline phosphatase gene: two kinds of mRNA from a single gene. Biochem Biophys Res Commun. 1990 May 16;168(3):993–1000. doi: 10.1016/0006-291x(90)91127-e. [DOI] [PubMed] [Google Scholar]
- Millán J. L., Manes T. Seminoma-derived Nagao isozyme is encoded by a germ-cell alkaline phosphatase gene. Proc Natl Acad Sci U S A. 1988 May;85(9):3024–3028. doi: 10.1073/pnas.85.9.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minty A. J., Caravatti M., Robert B., Cohen A., Daubas P., Weydert A., Gros F., Buckingham M. E. Mouse actin messenger RNAs. Construction and characterization of a recombinant plasmid molecule containing a complementary DNA transcript of mouse alpha-actin mRNA. J Biol Chem. 1981 Jan 25;256(2):1008–1014. [PubMed] [Google Scholar]
- Mira-y-Lopez R. Retinoic acid priming potentiates the induction of urokinase-type plasminogen activator by cyclic adenosine monophosphate in mouse mammary carcinoma cells. J Cell Physiol. 1991 Apr;147(1):46–54. doi: 10.1002/jcp.1041470107. [DOI] [PubMed] [Google Scholar]
- Murtaugh M. P., Moore W. T., Jr, Davies P. J. Cyclic AMP potentiates the retinoic acid-induced expression of tissue transglutaminase in peritoneal macrophages. J Biol Chem. 1986 Jan 15;261(2):614–621. [PubMed] [Google Scholar]
- Nabeshima Y., Fujii-Kuriyama Y., Muramatsu M., Ogata K. Alternative transcription and two modes of splicing results in two myosin light chains from one gene. Nature. 1984 Mar 22;308(5957):333–338. doi: 10.1038/308333a0. [DOI] [PubMed] [Google Scholar]
- Nicholson R. C., Mader S., Nagpal S., Leid M., Rochette-Egly C., Chambon P. Negative regulation of the rat stromelysin gene promoter by retinoic acid is mediated by an AP1 binding site. EMBO J. 1990 Dec;9(13):4443–4454. doi: 10.1002/j.1460-2075.1990.tb07895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson I. L., Breitman T. R. Induction of differentiation of the human histiocytic lymphoma cell line U-937 by retinoic acid and cyclic adenosine 3':5'-monophosphate-inducing agents. Cancer Res. 1982 Oct;42(10):3924–3927. [PubMed] [Google Scholar]
- Petkovich M., Brand N. J., Krust A., Chambon P. A human retinoic acid receptor which belongs to the family of nuclear receptors. Nature. 1987 Dec 3;330(6147):444–450. doi: 10.1038/330444a0. [DOI] [PubMed] [Google Scholar]
- Rambaldi A., Young D. C., Griffin J. D. Expression of the M-CSF (CSF-1) gene by human monocytes. Blood. 1987 May;69(5):1409–1413. [PubMed] [Google Scholar]
- Reese D. H., Larsen R. A., Hornicek F. J. Control of alkaline phosphatase activity in C3H10T1/2 cells: role of retinoic acid and cell density. J Cell Physiol. 1992 May;151(2):239–248. doi: 10.1002/jcp.1041510204. [DOI] [PubMed] [Google Scholar]
- Reese D. H., Politano V. A. Evidence for the retinoid control of urothelial alkaline phosphatase. Biochem Biophys Res Commun. 1981 Sep 16;102(1):322–327. doi: 10.1016/0006-291x(81)91524-2. [DOI] [PubMed] [Google Scholar]
- Rocchetti M., Recchia M. SPBS: statistical programs for biological sciences. Minicomputer software for applying routine biostatistical methods. Comput Programs Biomed. 1982 Feb;14(1):7–20. doi: 10.1016/0010-468x(82)90084-8. [DOI] [PubMed] [Google Scholar]
- Seiler-Tuyns A., Birnstiel M. L. Structure and expression in L-cells of a cloned H4 histone gene of the mouse. J Mol Biol. 1981 Oct 5;151(4):607–625. doi: 10.1016/0022-2836(81)90426-5. [DOI] [PubMed] [Google Scholar]
- Sozzani S., Agwu D. E., McCall C. E., O'Flaherty J. T., Schmitt J. D., Kent J. D., McPhail L. C. Propranolol, a phosphatidate phosphohydrolase inhibitor, also inhibits protein kinase C. J Biol Chem. 1992 Oct 5;267(28):20481–20488. [PubMed] [Google Scholar]
- Strickland S., Mahdavi V. The induction of differentiation in teratocarcinoma stem cells by retinoic acid. Cell. 1978 Oct;15(2):393–403. doi: 10.1016/0092-8674(78)90008-9. [DOI] [PubMed] [Google Scholar]
- Strickland S., Smith K. K., Marotti K. R. Hormonal induction of differentiation in teratocarcinoma stem cells: generation of parietal endoderm by retinoic acid and dibutyryl cAMP. Cell. 1980 Sep;21(2):347–355. doi: 10.1016/0092-8674(80)90471-7. [DOI] [PubMed] [Google Scholar]
- Studer M., Terao M., Gianni M., Garattini E. Characterization of a second promoter for the mouse liver/bone/kidney-type alkaline phosphatase gene: cell and tissue specific expression. Biochem Biophys Res Commun. 1991 Sep 30;179(3):1352–1360. doi: 10.1016/0006-291x(91)91722-o. [DOI] [PubMed] [Google Scholar]
- Swarup G., Cohen S., Garbers D. L. Selective dephosphorylation of proteins containing phosphotyrosine by alkaline phosphatases. J Biol Chem. 1981 Aug 10;256(15):8197–8201. [PubMed] [Google Scholar]
- Takahashi N., Breitman T. R. Covalent binding of 17 beta-estradiol and retinoic acid to proteins in the human breast cancer cell line MCF-7. In Vitro Cell Dev Biol. 1989 Dec;25(12):1199–1200. doi: 10.1007/BF02621275. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Breitman T. R. Retinoic acid acylation (retinoylation) of a nuclear protein in the human acute myeloid leukemia cell line HL60. J Biol Chem. 1989 Mar 25;264(9):5159–5163. [PubMed] [Google Scholar]
- Takahashi N., Breitman T. R. Retinoylation of proteins in leukemia, embryonal carcinoma, and normal kidney cell lines: differences associated with differential responses to retinoic acid. Arch Biochem Biophys. 1991 Feb 15;285(1):105–110. doi: 10.1016/0003-9861(91)90334-f. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Liapi C., Anderson W. B., Breitman T. R. Retinoylation of the cAMP-binding regulatory subunits of type I and type II cAMP-dependent protein kinases in HL60 cells. Arch Biochem Biophys. 1991 Nov 1;290(2):293–302. doi: 10.1016/0003-9861(91)90544-s. [DOI] [PubMed] [Google Scholar]
- Terao M., Mintz B. Cloning and characterization of a cDNA coding for mouse placental alkaline phosphatase. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7051–7055. doi: 10.1073/pnas.84.20.7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terao M., Studer M., Gianní M., Garattini E. Isolation and characterization of the mouse liver/bone/kidney-type alkaline phosphatase gene. Biochem J. 1990 Jun 15;268(3):641–648. doi: 10.1042/bj2680641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaller C., Eichele G. Identification and spatial distribution of retinoids in the developing chick limb bud. Nature. 1987 Jun 18;327(6123):625–628. doi: 10.1038/327625a0. [DOI] [PubMed] [Google Scholar]
- Toh Y., Yamamoto M., Endo H., Misumi Y., Ikehara Y. Duplicate leader exons in the mouse liver-type alkaline phosphatase gene. Biochem Int. 1990 Oct;22(2):213–218. [PubMed] [Google Scholar]
- Toh Y., Yamamoto M., Endo H., Misumi Y., Ikehara Y. Isolation and characterization of a rat liver alkaline phosphatase gene. A single gene with two promoters. Eur J Biochem. 1989 Jun 15;182(2):231–237. doi: 10.1111/j.1432-1033.1989.tb14822.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasios G. W., Gold J. D., Petkovich M., Chambon P., Gudas L. J. A retinoic acid-responsive element is present in the 5' flanking region of the laminin B1 gene. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9099–9103. doi: 10.1073/pnas.86.23.9099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler-Guettler H., Yu K., Soff G., Gudas L. J., Rosenberg R. D. Thrombomodulin gene regulation by cAMP and retinoic acid in F9 embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2155–2159. doi: 10.1073/pnas.89.6.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss M. J., Henthorn P. S., Lafferty M. A., Slaughter C., Raducha M., Harris H. Isolation and characterization of a cDNA encoding a human liver/bone/kidney-type alkaline phosphatase. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7182–7186. doi: 10.1073/pnas.83.19.7182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss M. J., Ray K., Henthorn P. S., Lamb B., Kadesch T., Harris H. Structure of the human liver/bone/kidney alkaline phosphatase gene. J Biol Chem. 1988 Aug 25;263(24):12002–12010. [PubMed] [Google Scholar]
- Wood W. I., Gitschier J., Lasky L. A., Lawn R. M. Base composition-independent hybridization in tetramethylammonium chloride: a method for oligonucleotide screening of highly complex gene libraries. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1585–1588. doi: 10.1073/pnas.82.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelent A., Krust A., Petkovich M., Kastner P., Chambon P. Cloning of murine alpha and beta retinoic acid receptors and a novel receptor gamma predominantly expressed in skin. Nature. 1989 Jun 29;339(6227):714–717. doi: 10.1038/339714a0. [DOI] [PubMed] [Google Scholar]
- Zelent A., Mendelsohn C., Kastner P., Krust A., Garnier J. M., Ruffenach F., Leroy P., Chambon P. Differentially expressed isoforms of the mouse retinoic acid receptor beta generated by usage of two promoters and alternative splicing. EMBO J. 1991 Jan;10(1):71–81. doi: 10.1002/j.1460-2075.1991.tb07922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zernik J., Kream B., Twarog K. Tissue-specific and dexamethasone-inducible expression of alkaline phosphatase from alternative promoters of the rat bone/liver/kidney/placenta gene. Biochem Biophys Res Commun. 1991 May 15;176(3):1149–1156. doi: 10.1016/0006-291x(91)90405-v. [DOI] [PubMed] [Google Scholar]
- Zhang X. K., Hoffmann B., Tran P. B., Graupner G., Pfahl M. Retinoid X receptor is an auxiliary protein for thyroid hormone and retinoic acid receptors. Nature. 1992 Jan 30;355(6359):441–446. doi: 10.1038/355441a0. [DOI] [PubMed] [Google Scholar]