Abstract
The NO-releasing compounds 3-morpholinosydnonimine-N-ethylcarbamide (SIN-1), sodium nitroprusside (SNP) and S-nitroso-N-acetyl-DL-penicillamine (SNAP) mediated a rapid loss of viability of Fu5 rat hepatoma cells. SIN-1 in addition to NO also released the superoxide anion radical (O2-.). Its cytotoxicity, however, was not affected by superoxide dismutase. In contrast, the H2O2-converting enzyme catalase significantly, but not completely, diminished cell damage, indicating participation of H2O2 in the tumoricidal activity of SIN-1. Glucose oxidase (5 m-units/ml), producing similar amounts of H2O2 to 5 mM SIN-1, had no effect on cell viability. When 5 m-units/ml glucose oxidase was added to incubations with 5 mM SNP, which alone initiated cell injury of about 40%, cell damage was significantly increased up to 95%. Similar results were observed with 1 mM SNAP and 20 m-units/ml xanthine oxidase, which mediated cytotoxicity of about 90% when both compounds were added together, compared with 35% and 55% cell injury, respectively, induced by the single compounds. The results indicate that a co-operative action with H2O2 enhances the tumoricidal activity of NO in Fu5 cells. No evidence for an interplay of NO with O2-. in cytotoxicity, e.g. via the peroxynitrite anion (ONOO-), was found.
Full text
PDF

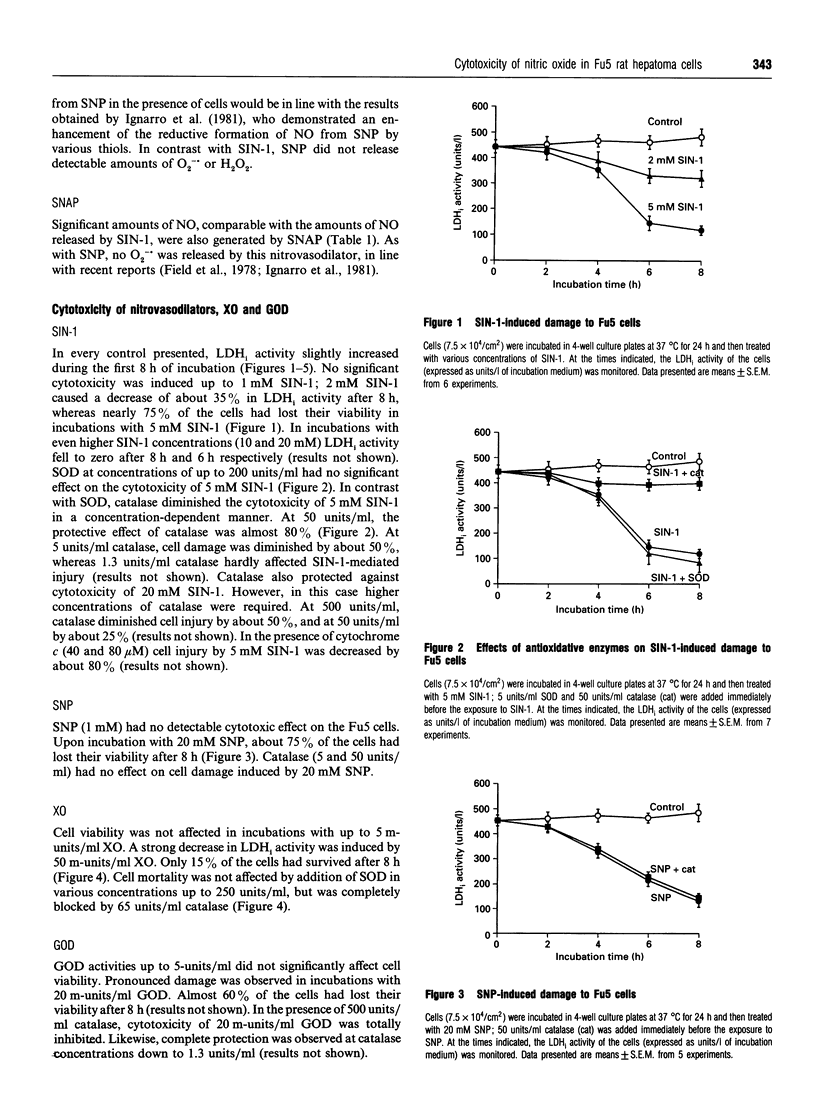
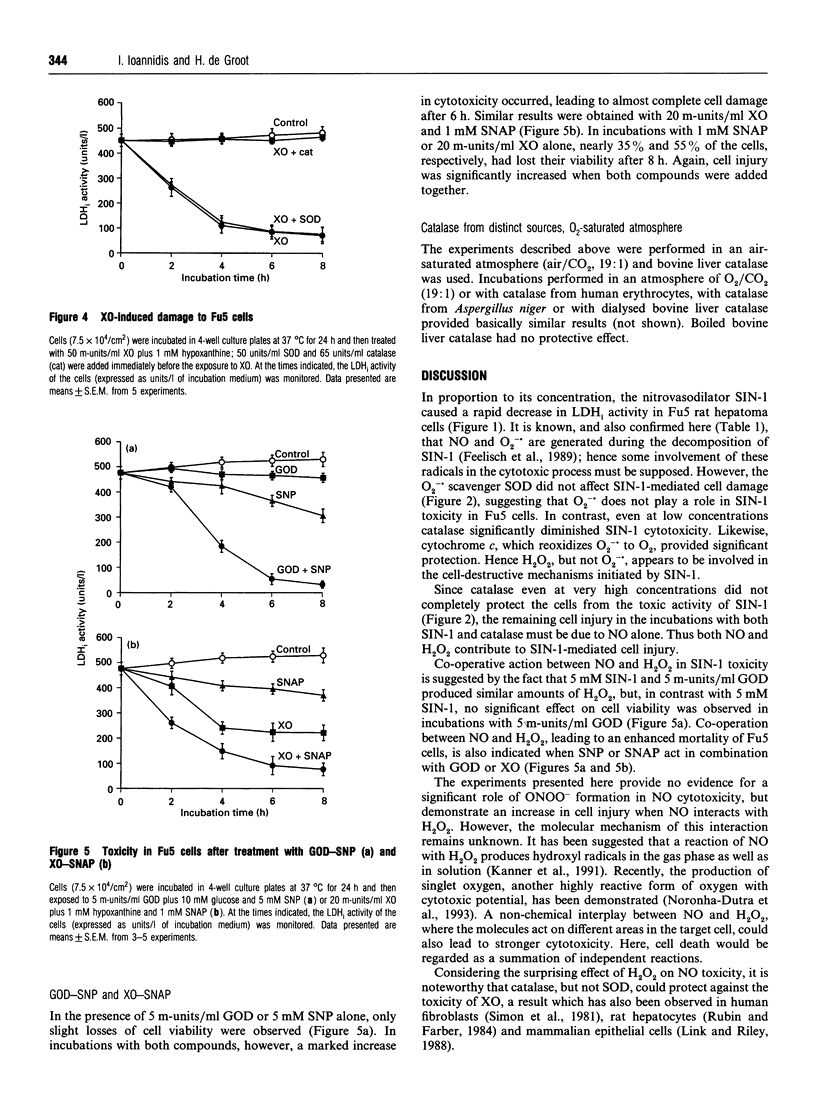

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckman J. S., Beckman T. W., Chen J., Marshall P. A., Freeman B. A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darley-Usmar V. M., Hogg N., O'Leary V. J., Wilson M. T., Moncada S. The simultaneous generation of superoxide and nitric oxide can initiate lipid peroxidation in human low density lipoprotein. Free Radic Res Commun. 1992;17(1):9–20. doi: 10.3109/10715769209061085. [DOI] [PubMed] [Google Scholar]
- Feelisch M., Noack E. A. Correlation between nitric oxide formation during degradation of organic nitrates and activation of guanylate cyclase. Eur J Pharmacol. 1987 Jul 2;139(1):19–30. doi: 10.1016/0014-2999(87)90493-6. [DOI] [PubMed] [Google Scholar]
- Feelisch M., Noack E. Nitric oxide (NO) formation from nitrovasodilators occurs independently of hemoglobin or non-heme iron. Eur J Pharmacol. 1987 Oct 27;142(3):465–469. doi: 10.1016/0014-2999(87)90090-2. [DOI] [PubMed] [Google Scholar]
- Fossati P., Prencipe L., Berti G. Use of 3,5-dichloro-2-hydroxybenzenesulfonic acid/4-aminophenazone chromogenic system in direct enzymic assay of uric acid in serum and urine. Clin Chem. 1980 Feb;26(2):227–231. [PubMed] [Google Scholar]
- Fridovich I. Quantitative aspects of the production of superoxide anion radical by milk xanthine oxidase. J Biol Chem. 1970 Aug 25;245(16):4053–4057. [PubMed] [Google Scholar]
- Green L. C., Wagner D. A., Glogowski J., Skipper P. L., Wishnok J. S., Tannenbaum S. R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982 Oct;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z., Rachlin E. M. Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem Biophys Res Commun. 1988 Nov 30;157(1):87–94. doi: 10.1016/s0006-291x(88)80015-9. [DOI] [PubMed] [Google Scholar]
- Hugo-Wissemann D., Anundi I., Lauchart W., Viebahn R., de Groot H. Differences in glycolytic capacity and hypoxia tolerance between hepatoma cells and hepatocytes. Hepatology. 1991 Feb;13(2):297–303. [PubMed] [Google Scholar]
- Ignarro L. J., Lippton H., Edwards J. C., Baricos W. H., Hyman A. L., Kadowitz P. J., Gruetter C. A. Mechanism of vascular smooth muscle relaxation by organic nitrates, nitrites, nitroprusside and nitric oxide: evidence for the involvement of S-nitrosothiols as active intermediates. J Pharmacol Exp Ther. 1981 Sep;218(3):739–749. [PubMed] [Google Scholar]
- Kanner J., Harel S., Granit R. Nitric oxide as an antioxidant. Arch Biochem Biophys. 1991 Aug 15;289(1):130–136. doi: 10.1016/0003-9861(91)90452-o. [DOI] [PubMed] [Google Scholar]
- Keller R., Keist R. Abilities of activated macrophages to manifest tumoricidal activity and to generate reactive nitrogen intermediates: a comparative study in vitro and ex vivo. Biochem Biophys Res Commun. 1989 Nov 15;164(3):968–973. doi: 10.1016/0006-291x(89)91764-6. [DOI] [PubMed] [Google Scholar]
- Link E. M., Riley P. A. Role of hydrogen peroxide in the cytotoxicity of the xanthine/xanthine oxidase system. Biochem J. 1988 Jan 15;249(2):391–399. doi: 10.1042/bj2490391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASSEY V. The microestimation of succinate and the extinction coefficient of cytochrome c. Biochim Biophys Acta. 1959 Jul;34:255–256. doi: 10.1016/0006-3002(59)90259-8. [DOI] [PubMed] [Google Scholar]
- Marletta M. A., Yoon P. S., Iyengar R., Leaf C. D., Wishnok J. S. Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry. 1988 Nov 29;27(24):8706–8711. doi: 10.1021/bi00424a003. [DOI] [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992 Sep;6(12):3051–3064. [PubMed] [Google Scholar]
- Noack E., Feelisch M. Molecular aspects underlying the vasodilator action of molsidomine. J Cardiovasc Pharmacol. 1989;14 (Suppl 11):S1–S5. [PubMed] [Google Scholar]
- Noronha-Dutra A. A., Epperlein M. M., Woolf N. Reaction of nitric oxide with hydrogen peroxide to produce potentially cytotoxic singlet oxygen as a model for nitric oxide-mediated killing. FEBS Lett. 1993 Apr 19;321(1):59–62. doi: 10.1016/0014-5793(93)80621-z. [DOI] [PubMed] [Google Scholar]
- Radi R., Beckman J. S., Bush K. M., Freeman B. A. Peroxynitrite-induced membrane lipid peroxidation: the cytotoxic potential of superoxide and nitric oxide. Arch Biochem Biophys. 1991 Aug 1;288(2):481–487. doi: 10.1016/0003-9861(91)90224-7. [DOI] [PubMed] [Google Scholar]
- Radi R., Cosgrove T. P., Beckman J. S., Freeman B. A. Peroxynitrite-induced luminol chemiluminescence. Biochem J. 1993 Feb 15;290(Pt 1):51–57. doi: 10.1042/bj2900051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin R., Farber J. L. Mechanisms of the killing of cultured hepatocytes by hydrogen peroxide. Arch Biochem Biophys. 1984 Feb 1;228(2):450–459. doi: 10.1016/0003-9861(84)90010-9. [DOI] [PubMed] [Google Scholar]
- Saran M., Bors W. Oxygen radicals acting as chemical messengers: a hypothesis. Free Radic Res Commun. 1989;7(3-6):213–220. doi: 10.3109/10715768909087944. [DOI] [PubMed] [Google Scholar]
- Schneider J. A., Weiss M. C. Expression of differentiated functions in hepatoma cell hybrids. I. Tyrosine aminotransferase in hepatoma-fibroblast hybrids. Proc Natl Acad Sci U S A. 1971 Jan;68(1):127–131. doi: 10.1073/pnas.68.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon R. H., Scoggin C. H., Patterson D. Hydrogen peroxide causes the fatal injury to human fibroblasts exposed to oxygen radicals. J Biol Chem. 1981 Jul 25;256(14):7181–7186. [PubMed] [Google Scholar]
- Wang J. F., Komarov P., Sies H., de Groot H. Contribution of nitric oxide synthase to luminol-dependent chemiluminescence generated by phorbol-ester-activated Kupffer cells. Biochem J. 1991 Oct 1;279(Pt 1):311–314. doi: 10.1042/bj2790311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. F., Komarov P., Sies H., de Groot H. Inhibition of superoxide and nitric oxide release and protection from reoxygenation injury by Ebselen in rat Kupffer cells. Hepatology. 1992 Jun;15(6):1112–1116. doi: 10.1002/hep.1840150623. [DOI] [PubMed] [Google Scholar]
- Wang J. F., Komarov P., de Groot H. Luminol chemiluminescence in rat macrophages and granulocytes: the role of NO, O2-/H2O2, and HOCl. Arch Biochem Biophys. 1993 Jul;304(1):189–196. doi: 10.1006/abbi.1993.1338. [DOI] [PubMed] [Google Scholar]
- de Groot H., Hegi U., Sies H. Loss of alpha-tocopherol upon exposure to nitric oxide or the sydnonimine SIN-1. FEBS Lett. 1993 Jan 4;315(2):139–142. doi: 10.1016/0014-5793(93)81150-x. [DOI] [PubMed] [Google Scholar]


