Fig. 2. Strategy for building α-helical backbone structure topologies.
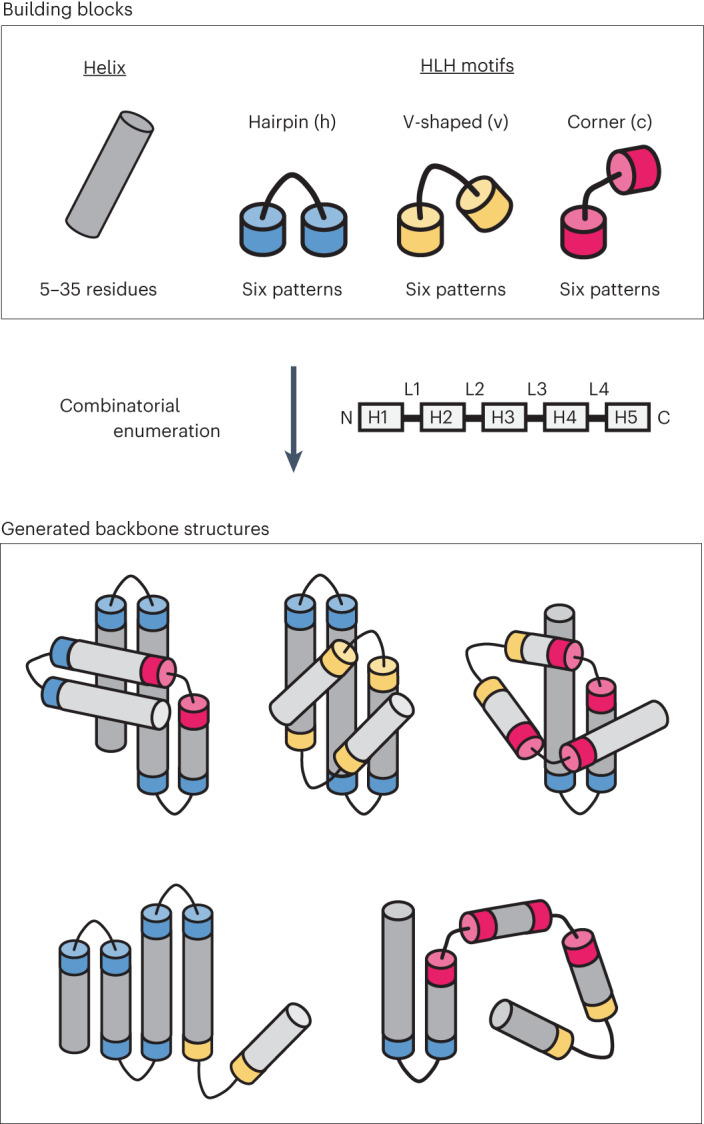
Top: building blocks for generating backbone structures. Canonical α-helices and three types of HLH tertiary motifs typically observed in nature, hairpin (h), v-shaped (v) and corner (c), are used. Helices range from 5 to 35 residues, and each motif type comprises six patterns (Fig. 3a). The motif types were classified on the basis of the bending angle between the constituent helices in HLH motifs. Middle: secondary-structure element ordering to build α-helical proteins with five helices. According to the ordering, globular backbone structures without steric clashes are exhaustively explored by combining the building blocks, with the constraint of total residue length. Bottom: examples for generated α-helical backbone structure topologies. Poorly packed structures (lower) are discarded, whereas globularly folded structures (upper) are collected.
