Abstract
To gain insight into avian influenza virus (AIV) transmission, exposure, and maintenance patterns in shorebirds at Delaware Bay during spring migration, we examined temporal AIV prevalence trends in four Charadriiformes species with the use of serial cross-sectional data from 2000 through 2008 and generalized linear and additive models. Prevalence of AIV in Ruddy Turnstones (Arenaria interpres morinella) increased after arrival, peaked in mid-late May, and decreased prior to departure. Antibody prevalence also increased over this period; together, these results suggested local infection and recovery prior to departure. Red Knots (Calidris canutus rufa), Sanderlings (Calidris alba), and Laughing Gulls (Leucophaeus atricilla) were rarely infected, but dynamic changes in antibody prevalence differed among species. In Red Knots, declining antibody prevalence over the stopover period suggested AIV exposure prior to arrival at Delaware Bay with limited infection at this site. Antibody prevalence was consistently high in Laughing Gulls and low in Sanderlings. Both viral prevalence and antibody prevalence in Sanderlings varied directly with those in turnstones, suggesting virus spillover to Sanderlings. Results indicate that, although hundreds of thousands of birds concentrate at Delaware Bay during spring, dynamics of AIV infection differ among species, perhaps due to differences in susceptibility, potential for contact with AIV at this site, or prior exposure. Additionally, Ruddy Turnstones possibly act as a local AIV amplifying host rather than a reservoir.
Keywords: AIV, avian influenza virus, Charadriiformes, Delaware Bay, disease ecology, infection dynamics, Ruddy Turnstone (Arenaria interpres morinella), shorebird
INTRODUCTION
Although Anseriformes (swans, geese, and ducks) and Charadriiformes (gulls and shorebirds) are the natural reservoirs of avian influenza viruses (AIV), our current understanding of AIV epidemiology in these reservoirs is limited by the patchy nature of surveillance across space, time, and species (Stallknecht and Brown, 2007). Among ducks, AIV prevalence typically peaks in late summer and autumn as birds, particularly nïve juveniles, aggregate on premigratory staging grounds (Olsen et al., 2006). Some species of Charadriiformes, such as gulls (family Laridae) and shorebirds (families Charadriidae and Scolopacidae), also are important in global AIV epidemiology, and certain subtypes are associated with and are particularly well adapted to gull species (Hinshaw et al., 1983; Yamnikova et al., 2003; Fouchier et al., 2005). Although transmission among ducks occurs primarily through fecally contaminated water (Hinshaw et al., 1979), transmission and maintenance among Charadriiformes species is poorly understood.
Initial studies conducted at Delaware Bay on the Atlantic coast of North America reported that AIV prevalence was 2.4–20% among sampled shorebirds and gulls in spring compared to 3.5–8% in autumn and 0% in winter and summer (Kawaoka et al., 1988). Estimated mean springtime prevalence among shorebirds and gulls was 6.3% across 21 yr (Krauss et al., 2010). Both studies recognized one shorebird species, the Ruddy Turnstone (Arenaria interpres morinella), as disproportionately infected, and Hanson et al. (2008) reported that mean springtime prevalence was significantly higher among Ruddy Turnstones (11%) than among 10 other species (0.5%). Despite this consistent seasonal pattern of infection among shorebirds at Delaware Bay, AIV have not been detected more than occasionally in shorebirds at other times and locations, particularly in the western hemisphere (e.g., Escudero et al., 2008; Iverson et al., 2008; Winker et al., 2008; Ghersi et al., 2009).
An estimated >1 million shorebirds use Delaware Bay each spring (Clark et al., 1993), where, by feasting on eggs of horseshoe crabs (Limulus polyphemus) on spawning beaches, they rapidly gain up to 70% of their arrival body masses to fuel long-distance flights to breeding grounds in the Arctic (Robinson et al., 2003). Each year, shorebirds begin arriving in early May following migrations from wintering and stopover areas in South America and the Caribbean (Myers et al., 1990; Morrison and Harrington, 1992) and depart for breeding grounds during late May–early June (Robinson et al., 2003). The stopover period is approximately 5 wk, although individual birds can remain for shorter periods (Gillings et al., 2009). From 12% to 80% of the North American populations of six species use Delaware Bay in spring, including Red Knots (Calidris canutus rufa), Ruddy Turnstones, Sanderlings (Calidris alba), Semipalmated Sandpipers (Calidris pusilla), Dunlins (Calidris alpina), and Short-billed Dowitchers (Limnodromus griseus) (reviewed by US Fish and Wildlife Service, 2003). Additionally, adjacent salt marshes in New Jersey support breeding colonies of Laughing Gulls (Leucophaeus atricilla), Herring Gulls (Larus argentatus), and Great Black-backed Gulls (Larus marinus), and non-breeding Ring-billed Gulls (Larus delawarensis) that also feed on horseshoe crab eggs alongside shorebirds (Burger et al., 2007).
Delaware Bay is adjacent to the Delmarva Peninsula, an important poultry-producing region. Because transmission of AIV from wild birds to poultry occasionally occurs with appropriate contact (Spackman, 2009), understanding the scale, scope, and timing of AIV epidemics in nearby wild bird populations is important to address this risk.
Our objectives were to describe temporal patterns of AIV prevalence and antibody prevalence within three shorebird species (Ruddy Turnstones, Red Knots, and Sanderlings) and one gull species (Laughing Gulls) during the spring migratory stopover at Delaware Bay, and compare patterns between species. Such information provided insight into possible AIV sources, transmission, exposure, and maintenance patterns at this location, as well as potential exposures outside of the Delaware Bay stopover.
MATERIALS AND METHODS
Field and laboratory methods
Fieldwork was conducted during 17–24 May 2006, 10 May–3 June 2007, and 7 May–4 June 2008 at Delaware Bay (39°N, 75°W). Shorebirds and gulls were captured with cannon nets as part of long-term population studies. Following banding and measurement, swab samples for virus isolation were collected, stored, processed, and tested in embryonated chicken eggs as previously described (Hanson et al., 2008). Cloacal swabs were collected from all birds; in addition, oropharyngeal swabs were collected from Laughing Gulls in 2008 and analyzed separately (none were positive). Fresh Laughing Gull feces were swabbed in limited cases and only from within the borders of monospecific breeding colonies. Presence of AIV was confirmed by hemagglutination (Swayne et al., 1998) and reverse transcriptase polymerase chain reaction (RT-PCR) for matrix gene (Spackman and Suarez, 2008) on allantoic fluid. All isolates were low pathogenic (LPAI) viruses (unpublished data).
During 2007–2008, blood samples were collected by jugular venipuncture from a random subset of swabbed birds. Collected volume ranged from <0.5% to 1% of a bird’s body mass in grams. Samples were kept on ice in the field and sera were stored at −20 C until testing. Antibodies against AIV nucleoprotein (NP) were tested with a commercial blocking enzyme-linked immunoassay (bELISA; FlockChek AI MultiS-Screen Antibody Test Kit, IDEXX Laboratories, Westbrook, Maine, USA). Samples yielding S/N ratios of <0.5 were considered positive, per manufacturer’s recommendations.
Morphometric data for individual sampled birds were obtained from the Shorebird Resighting Database (http://www.bandedbirds.org). Research was conducted under University of Georgia Animal Care and Use Committee approval and state and federal scientific collection permits.
Statistical analyses
Computations and statistical analyses were performed in JMP version 8 (SAS Institute Inc., Cary, North Carolina, USA). Virus isolation data from four species (Ruddy Turnstone, Red Knot, Sanderling, and Laughing Gull) were evaluated for temporal trends within stopover seasons. Data from 2006–2008 were pooled with results from 2000–2005 originally reported in Hanson et al. (2008). For each species, data from all 9 yr (4 yr for Laughing Gulls; 2005–2008) were used to estimate the average trend, if any. Additionally, the sampling date range spanned the majority of the stopover season in 2002, 2007, and 2008 (25, 25, and 29 days, respectively) and allowed evaluation of each of these years separately. Within a logit-link generalized linear model (GLM) framework, changes in prevalence were modeled as a linear, quadratic, or three-node knotted spline (Hastie and Tibshirani, 1990) function of date, to obtain maximum model fit. Because shorebirds are expected to gain mass over the stopover period, the above analyses were repeated with masses of sampled birds (available 2006–2008). Laughing Gulls were not weighed at the time of sampling and were excluded from these analyses. In some cases several models adequately described the prevalence trend; however, we present the single model that minimized Akaike’s Information Criterion () (Burnham and Anderson, 2002).
We also evaluated trends in antibody prevalence over time and over mass gain in each species with the use of GLM. Because the bELISA imperfectly detects recent AIV infection in wild bird species (test sensitivity=0.754, specificity=1; Brown et al., 2009), antibody prevalence calculations were adjusted according to Rogan and Gladen (1978):
where antibody prevalence, antibody prevalence, specificity, and sensitivity. Thus, the adjusted antibody prevalence for each day or 10-g mass class (weighted by ) was used rather than raw antibody status of individual birds. Serology data were pooled by shorebird species because temporal patterns did not differ between years (year effect with day as covariate, for Ruddy Turnstones: , , ; Red Knots: , , ; Sanderlings: , , ), but were examined for 2007–2008 separately in Laughing Gulls because the pattern varied between years (, , ). For Ruddy Turnstones, an estimate of the minimum proportion of birds that were exposed to AIV (i.e., seroconverted) during the stopover period was determined by subtracting the estimated antibody prevalence upon arrival at Delaware Bay from that upon departure.
Nonparametric Kendall’s correlation was used to assess the relationship of AIV prevalence on a given year and day between species pairs. To reduce measurement bias, we included data only when n≥5 simultaneously for both species. We further used logistic regression to examine the relationship between isolating any AIV in a species (i.e., AIV presence/absence) and prevalence in Ruddy Turnstones on that date. Here, only data when n≥30 were used.
RESULTS
Prevalence of AIV infection (Table 1) and AIV antibodies (Table 2) varied by species; both infection and antibody prevalence were highest in Ruddy Turnstones and lowest in Sanderlings. Red Knots and Laughing Gulls both exhibited high antibody prevalence but low infection prevalence. Annual AIV prevalence in Ruddy Turnstones was 10–20 times greater than in Red Knots and Sanderlings and 5–6 times greater than in Laughing Gulls.
Table 1.
Springtime stopover site avian influenza virus prevalence among four species of Charadriiformes at Delaware Bay, 2000–2008.
| Ruddy Turnstone |
Red Knot |
Sanderling |
Laughing Gull |
|||||
|---|---|---|---|---|---|---|---|---|
| Year | n (positive) | Prevalence (%) (95% confidence interval [CI]) | n (positive) | Prevalence (%) (95% CI) | n (positive) | Prevalence (%) (95% CI) | n (positive) | Prevalence (%) (95% CI) |
|
| ||||||||
| 2002 | 736 (75) | 10 (8.2–13) | 364 (2) | 0.5 (0.2–2.0) | 248 (2) | 0.8 (0.2–2.9) | n/aa | n/aa |
| 2007 | 415 (19) | 4.6 (3.0–7.0) | 242 (1) | 0.4 (0.1–2.3) | 191 (0) | 0.0 (0.0–2.0) | 48 (0) | 0.0 (0.0–7.4) |
| 2008 | 582 (97) | 17 (14–20) | 277 (2) | 0.7 (0.2–3.0) | 225 (1) | 0.4 (0.1–2.5) | 149 (4)b | 2.7 (1.0–6.7) |
| 2000–2008 | 3,660 (431) | 12 (12–13) | 2,438 (21) | 0.9 (0.6–1.3) | 1,269 (15) | 1.2 (0.7–1.9) | 332 (6)a | 1.8 (0.8–3.9)a |
Laughing Gulls were sampled during 2005–2008 only.
Two cloacal swabs and two fecal swabs positive.
Table 2.
Springtime stopover site avian influenza virus antibody prevalence among four species of Charadriiformes at Delaware Bay, 2007–2008.
| Species | Year | n (positive) | Calculated antibody prevalence (%) | Adjusted antibody prevalence (%)a (95% CI) |
|---|---|---|---|---|
|
| ||||
| Ruddy Turnstone | 2007–2008 | 380 (243) | 64 | 85 (78–91) |
| Red Knot | 2007–2008 | 211 (125) | 59 | 79 (70–87) |
| Sanderling | 2007–2008 | 153 (4) | 2.6 | 3.4 (0.1–6.8) |
| Laughing Gull | 2007 | 31 (23) | 74 | 98 (78–100) |
| 2008 | 52 (25) | 48 | 64 (46–82) | |
Adjusted for test sensitivity=0.754 (Brown et al., 2009), according to Rogan and Gladen (1978).
AIV dynamics in Ruddy Turnstones
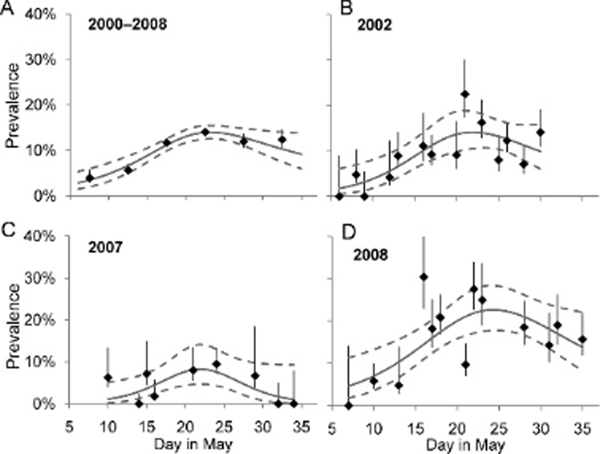
Prevalence of AIV varied with time similarly for 2000–2008 pooled data (three-node knotted spline fit: , , P<0.0001) and in each individual year (2002: , , ; 2007: , , ; 2008: , , ; Fig. 1). Although peak prevalence varied among years, peaks occurred within 2 calendar days of each other (Table 3). In all years, prevalences among birds sampled during periods of arrival and departure (<15 May or >May 30; Robinson et al., 2003) were lower than among birds sampled during 15–30 May (2002: 4.7% vs. 12%, , , ; 2007: 1.2% vs. 6.9%, , , ; 2008: 11% vs. 20%, , , ).
Figure 1.
Avian influenza virus prevalence in Ruddy Turnstones over the course of the spring stopover at Delaware Bay, (A) for 2000–2008 pooled data, and for each year sufficient serial data were available: (B) 2002, (C) 2007, and (D) 2008. Lines of fit (95% confidence interval) and prevalence (±SE) for each day (B, C, and D) or 5-day time span (A) when n≥5 are shown. All lines of fit are significant at α=0.05.
Table 3.
Estimated peak avian influenza virus prevalence by day and body mass in Ruddy Turnstones, 2000–2008, Delaware Bay. See text for details of model fit.
| By day |
By massa |
|||
|---|---|---|---|---|
| Year | Peak prevalence (%) (95% confidence interval [CI]) | Date of peak prevalence | Peak prevalence (%) (95% CI) | Mass at peak prevalence (g) |
|
| ||||
| 2002 | 14 (11–19) | 22 May | – | – |
| 2007 | 8.2 (4.7–14) | 22 May | 9.3 (5.3–16) | 133 |
| 2008 | 23 (18–28) | 24 May | 24 (18–30) | 132 |
| 2000–2008 | 13 (11–14) | 24 May | – | – |
| 2006–2008 | – | – | 19 (16–22) | 134 |
Mass data were not available prior to 2006.
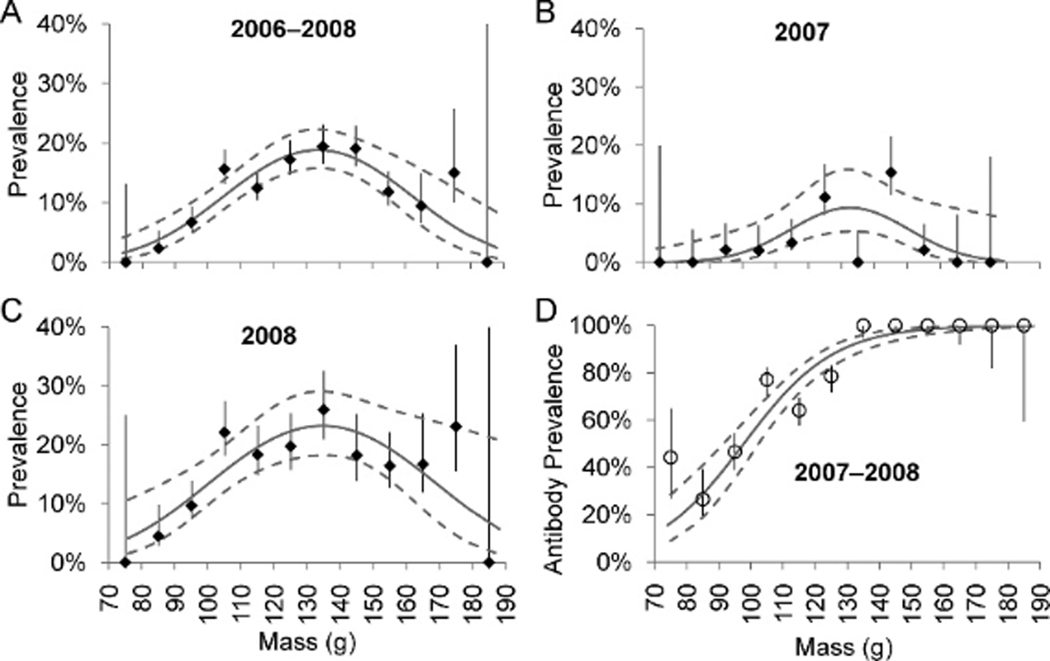
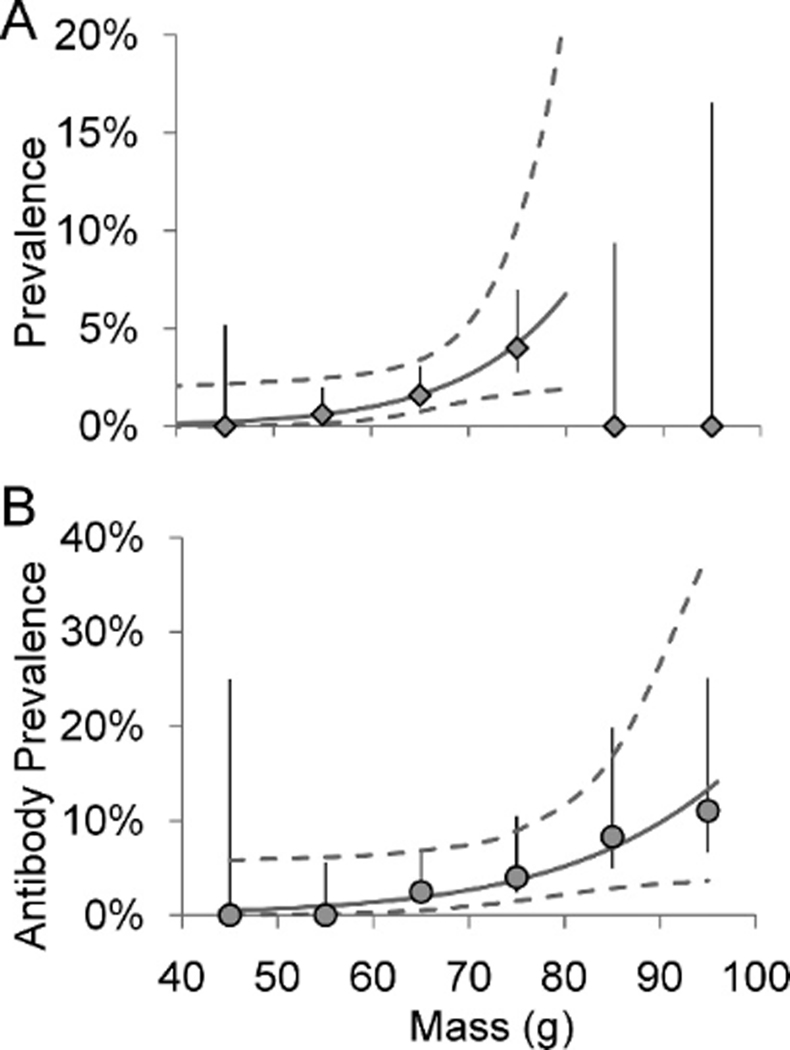
Estimated AIV prevalence also varied over mass, both for 2006–2008 pooled data (quadratic fit, , , P<0.0001) and in individual years (2007: quadratic fit, , , ; 2008: three-node knotted spline fit, , , ; Fig. 2). In both 2007 and 2008, prevalence was lower among birds with masses suggesting recent arrival or impending departure (≤99 or ≤157g; Robinson et al., 2003) than among birds with midrange masses (2007: 0.8% vs. 6.3%, , , ; 2008: 12% vs. 20%, , , ). Mass associated with peak prevalence varied by only 2 g in different years (Table 3).
Figure 2.
Avian influenza virus prevalence during (A) 2006–2008, (B) 2007, and (C) 2008, and (D) antibody prevalence during 2007–2008 in Ruddy Turnstones by 10-g mass classes, Delaware Bay. Lines of fit and 95% confidence interval are shown (all fits are significant at α=0.01). Also shown are prevalences and adjusted antibody prevalences (±SE) positioned at the midpoint of each class (e.g., adjusted antibody prevalence among birds 90–99 g is plotted at 95 g).
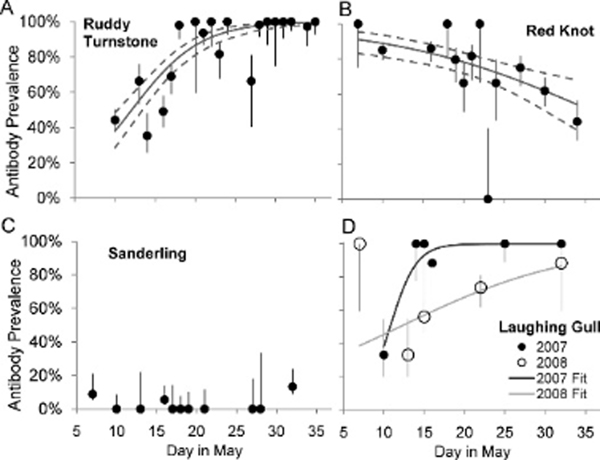
Adjusted antibody prevalence increased over the stopover season (, , P<0.0001; Fig. 3a) from <40% positive on 10 May to >95% positive by 25 May. Adjusted antibody prevalence was lower among birds sampled on or before 17 May, corresponding to the rapid prevalence increase (Fig. 1), than among birds sampled later (51% vs. 100%;, , P<0.0001). Adjusted antibody prevalence also increased with mass (, , P<0.0001). Antibody prevalence among birds weighing ≤99 g was significantly lower than among heavier birds (41% vs. 91%;, , P<0.0001), and antibody prevalence among birds weighing ≥157 g was higher than among lighter birds (100% vs. 82%;, , ).
Figure 3.
Estimated AIV antibody prevalence (95% CI) over the stopover season at Delaware Bay in (A) Ruddy Turnstones, (B) Red Knots, (C) Sanderlings, and (D) Laughing Gulls. Also shown are adjusted antibody prevalences (±SE) on each sample date (data from both years combined for Ruddy Turnstones, Red Knots, and Sanderlings). All fits are significant at .
Evidence and estimates of seroconversion:
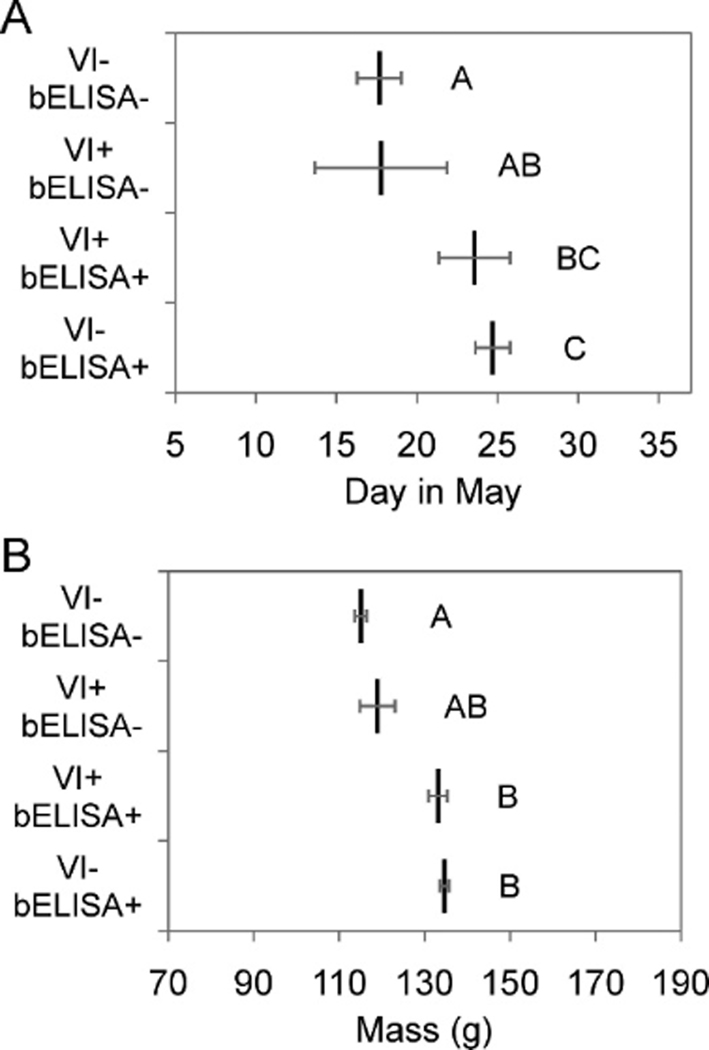
Individual Ruddy Turnstones that were tested both by VI and bELISA () were divided into four categories based on results of the two tests. Both the mean sample date and mean mass varied across categories (one-way analysis of variance [ANOVA]; date: , , P<0.0001; mass: , , P<0.0001) and increased in the order: VI negative and bELISA negative, VI positive and bELISA negative, VI positive and bELISA positive, VI negative and bELISA positive (Fig. 4). Migrants typically arrive at Delaware Bay asynchronously during late April–mid-May, but depart en masse over a few days in late May–early June (Clark et al., 1993; Gillings et al., 2009). Assuming that arriving birds have similar masses regardless of arrival date (Gillings et al., 2009), we compared the expected antibody prevalence among Ruddy Turnstones at the mean arrival mass (96 g; Niles et al. unpublished data, cited in US Fish and Wildlife Service, 2003) to the estimated antibody prevalence on the mean departure date (1 June; unpublished data), or 48% and 99%, respectively. Thus, approximately 51% of the Ruddy Turnstone population seroconverted during the stopover.
Figure 4.
Mean (±SE) (A) sample date and (B) mass of Ruddy Turnstones testing positive (+) or negative (−) on virus isolation (VI) and serology (bELISA). Within each panel, the mean sample date or mass among birds belonging to categories not connected by the same letter are significantly different (Tukey–Kramer post hoc tests; α=0.05).
AIV dynamics in Red Knots
AIV prevalence in Red Knots remained low (<2%) over the stopover period during each year and was not associated with mass (2006–2008 pooled data;, , ; data not shown). In contrast to Ruddy Turnstones, adjusted antibody prevalence in Red Knots decreased significantly over time (, , ; Fig. 3b). Antibody prevalence varied with 10-g mass class in a quadratic manner (, , ), and was higher among midweight birds than among birds with either near-arrival (≤114 g) or near-departure (≥176 g) masses (82% vs. 70%;, , ).
AIV dynamics in Sanderlings
Viral prevalence increased with day during 2000–2008 (, , ) but not within any individual year (all P>0.05; data not shown). All Sanderling AIV isolations occurred during 17–30 May (prevalence=1.5%); this prevalence was significantly higher than the 0% prevalence during the arrival period (, , ) but not the departure period (P>0.05). Prevalence did not vary with mass, either for pooled 2006–2008 data or during 2008 alone (all P>0.05). However, a significant trend was observed when birds weighing >80 g (representing <5% of tested birds) were excluded (, , ; Fig. 5a). Prevalences among arrival-(≤53 g), intermediate-(54–90 g), and departure-weight (≥91 g) birds were 1.3%, 1.6%, and 0%, respectively, and were not statistically different (, , ).
Figure 5.
Avian influenza virus (A) prevalence (2006–2008) and (B) antibody prevalence by 10-g mass class (2007–2008) in Sanderlings. Lines of fit and 95% confidence interval are shown (all fits are significant at α=0.05); see text for details.
Adjusted antibody prevalence did not vary over time (, , ; Fig. 3c) but increased with mass (, , ; Fig. 5b). Adjusted antibody prevalence did not differ among arrival-, midrange, and departure-weight birds (0%, 3.2%, and 12%, respectively;, , ). However, sample sizes were and in arrival- and departure-weight groups, respectively.
AIV dynamics in Laughing Gulls
Infection prevalence did not vary temporally within a stopover season during 2005–2008 or the individual years 2007 or 2008 (all P>0.05; data not shown). Adjusted daily antibody prevalences increased with time and were higher in 2007 than in 2008 (Fig. 3d); a significant year × day interaction was present (whole model: , , ; day effect: , , ; year effect: , , ; year×day effect: , , ).
AIV prevalence correlations between species
Prevalence among Sanderlings was positively correlated with prevalence among Ruddy Turnstones captured on the same day ( catches, Kendall’s , ). Daily prevalences among Red Knots and Laughing Gulls were not correlated with prevalence among Ruddy Turnstones (, , ; and , , , respectively), nor were prevalences correlated among Sanderlings, Red Knots, and Laughing Gulls (all P>0.05). Additionally, the probability of detecting AIV among a sample of ≥30 Sanderlings was positively associated with AIV prevalence among Ruddy Turnstones on that day (logistic regression: , , ) and was likely (≥50% probability) when Ruddy Turnstone prevalence exceeded 18.6%. There was no association between prevalence among Ruddy Turnstones and AIV presence among Red Knots (, , ) or Laughing Gulls (, , ) on a given date.
DISCUSSION
Ruddy Turnstones
There are few reports of AIV in Ruddy Turnstones at times and locations outside the Delaware Bay spring migratory stopover period. The consistent temporal pattern of infection and seroconversion (between and within years) at this site suggests an annual and localized epidemic; reported AIV isolations from Ruddy Turnstones at Delaware Bay each May since 1985 (Krauss et al., 2004; Hanson et al., 2008; Krauss et al., 2010) clearly demonstrate this predictable event.
All plots of AIV prevalence over time and mass gain had similar patterns of near-zero prevalence during early May when turnstones are at arrival weights, followed by abrupt increases in prevalence on approximately 16–17 May as the season progressed and birds gained weight. Antibody prevalence also increased during each stopover season; together these measures indicate that most exposures and infections occurred after arrival at Delaware Bay. The AIV infection duration is unknown in shorebirds, but the narrowness of the epidemic curve suggests short periods of shedding similar to the 2–8 days reported in wild Mallards (Latorre-Margalef et al., 2009). Infections decreased after 24 May, possibly due to an increase in population immunity. In 2007, the entire epidemic lasted less than 25 days before a complete fadeout, but in 2008 it lasted longer. We did not sample after 4 June because the small remaining number of birds were more dispersed and difficult to capture, but these data would have been useful to accurately determine the epidemic’s span.
Similarly, patterns of antibody prevalence indicated population seroconversion in the wake of an epidemic. Generally, individual birds were virus- and antibody-negative at the beginning of the stopover, became infected, seroconverted within a few days of infection, and then recovered from infection but retained circulating antibodies. This pattern is expected with an acute infectious disease (Nunn and Altizer, 2006). We estimate that at least half of birds seroconverted during the stopover; this figure could include both previously unexposed birds and birds that had been previously exposed (at Delaware Bay or elsewhere) but whose antibodies had fallen below detectable levels (i.e., they were re-exposed at Delaware Bay). Because virtually all turnstones were antibody positive just prior to departure, the population proportion exposed at the stopover is likely higher than the above estimate.
Although the conditions that enhance AIV transmission in Ruddy Turnstones at Delaware Bay each spring are currently unknown, the high population density (up to 67 birds/m2; Gillings et al., 2007) of this species at Delaware Bay is an obvious and unique situation; such densities are not encountered during other times in their annual cycle (Nettleship, 2000). The sudden aggregation of susceptible Ruddy Turnstones at Delaware Bay could provide the population threshold (Lloyd-Smith et al., 2005) needed to initiate and sustain annual AIV epidemics (Krauss et al., 2010). Up to 80% of the A. interpres morinella population migrates through Delaware Bay each year (Morrison et al., 2001), and aerial counts have exceeded 100,000 turnstones on a single day (Clark et al., 1993). During the breeding, fall migration, and wintering periods Ruddy Turnstones are typically much more dispersed, often seen individually or in small flocks (n<50; Nettleship, 2000). Thus, potential bird-to-bird transmission of AIV could be interrupted when they do not regularly encounter a large number of conspecifics.
A majority of turnstones did not have detectable antibodies against AIV upon arrival at Delaware Bay. Though the duration for which anti-NP antibodies can be detected following initial exposure is unknown, it seems reasonable that the majority of Ruddy Turnstones had not been recently exposed to AIV, and indeed might never have been exposed (e.g., young adult birds migrating through Delaware Bay for the first time) or were last exposed during the preceding spring. Further, previous population exposure might have involved different AIV subtypes to which detected antibodies were not protective or only partially protective.
In addition to population density and immunity, increased potential virus contact at Delaware Bay due to the local density of other AIV reservoir species also should be considered. The largest Laughing Gull breeding colony on the Atlantic coast and smaller colonies of Herring Gulls, Great Black-backed Gulls, egrets, herons, and other waterbirds are located in close proximity on the Cape May Peninsula in New Jersey, as are migrant and resident waterfowl. Laughing and Herring Gulls number tens of thousands of breeding pairs (Pierotti and Good, 1994; Burger, 1996) and often feed alongside shorebirds on the beaches (Burger et al., 2007). Gulls are recognized AIV reservoirs and transmission might be associated with breeding behavior (Velarde et al., 2010). The high prevalence of AIV antibodies detected in gulls in the present study supports their possible involvement. Infection data in gulls, waterfowl, and other resident waterbirds prior to shorebird arrival would help determine if epidemiologic links exist between species groups.
Two additional unique and poorly understood factors that might enhance AIV transmission or susceptibility at this site are environmental conditions that allow effective exposure to and transmission of AIV and increased susceptibility related to the physiologic changes associated with long-distance migration and rapid weight gain. Migration and refueling at stopovers are physiologically stressful activities, and stress hormones such as corticosterone that could be important to shorebird stopover physiology (Piersma et al., 2000; Mizrahi et al., 2001) might also be immunosuppressive.
Because the majority of Ruddy Turnstones are infected with and recover from AIV during the spring stopover, and from Delaware Bay disperse onto the breeding grounds and remain dispersed during fall migration and winter, it is possible that AIV infection does not persist year-round in this species. This is supported by the low antibody prevalence we observed on arrival. Rather than being an AIV reservoir, which implies endemicity, it is possible that Ruddy Turnstones are a local amplifying host under conditions of high density, low flock immunity, and increased exposure to AIV. Similar conditions might exist at other locations involving Ruddy Turnstones or another permissive species; in October 2007, AIV were detected in Ruddy Turnstones in Peru (Ghersi et al., 2009). Although shorebirds have been implicated in rare transhemispheric movement of AIV gene segments (Krauss et al., 2007), a recent analysis suggests that AIV are more likely to be carried long distances by gulls and that shorebirds, such as Ruddy Turnstones, are probably local secondary hosts (Pearce et al., 2010).
Other species
Of the three syntopic species studied, only Sanderlings exhibited dynamical changes in AIV prevalence over the stopover period. Prevalence increased with day (≤30 May), and with mass (≤80 g), and was positively correlated with prevalence in Ruddy Turnstones. Sanderlings appear relatively resistant to AIV infection (perhaps due to decreased susceptibility or limited contact), given low prevalence and antibody prevalence at Delaware Bay, and zero prevalence at other times and locations (Hlinak et al., 2006; Munster et al., 2007; Winker et al., 2007; Escudero et al., 2008; Hanson et al., 2008; Iverson et al., 2008; Ghersi et al., 2009). Because infections in Sanderlings are sporadic and likely depend on the infected proportion of syntopic birds, they are probably due to spillover events from Ruddy Turnstones acting as amplifying hosts (Fenton and Pedersen, 2005).
The high antibody prevalence in Red Knots, which declined over the season, was an unexpected finding. Red Knots are not often found infected at Delaware Bay or the limited number of other locations where they have been sampled (D’Amico et al., 2007; Hanson et al., 2008). Although the duration of antibody detectability is unknown, this pattern suggests that Red Knots were exposed to AIV recently prior to arrival at Delaware Bay, perhaps on their wintering grounds or at another stopover during northward migration. Red Knots often winter in large flocks at Tierra del Fuego in Argentina and Chile and congregate at several stopovers prior to Delaware Bay (Harrington, 2001); therefore, opportunity could exist for AIV spread within localized populations throughout much of the year. Although AIV has not been detected on wintering grounds, available information is limited. More information regarding when and where Red Knots are exposed or infected would be helpful to understand what role, if any, LPAI infection has had on recent population declines (Baker et al., 2004; Niles et al., 2009).
High antibody prevalence could also result from long-lasting immunity following prior, or repeated, exposure(s) at Delaware Bay (Buehler et al., 2010). However, antibody prevalence declines rather than remaining high over the stopover duration, suggesting that Red Knots are not re-exposed to AIV while at Delaware Bay. This pattern is difficult to understand because knots and turnstones feed together on the Bay beaches. Perhaps subtle differences in host species ecology support transmission of virus among turnstones but not to knots. For example, Red Knots primarily consume horseshoe crab eggs available at the surface, but Ruddy Turnstones regularly dig several centimeters deep to reach buried egg masses (Tsipoura and Burger, 1999). If fecally excreted virus becomes rapidly unavailable on the beach surface (e.g., through UV irradiation or percolation into the sand due to wave action) but remains infectious when adsorbed to subsurface sand particles (Jin et al., 1997) or horseshoe crab eggs, then Red Knots might encounter viable AIV less often than Ruddy Turnstones. Sanderlings and gulls often raid turnstone excavations, whereas Red Knots rarely do (Burger et al., 2007; Vahl et al., 2007), perhaps helping to explain why Sanderlings and gulls are infected more often. Other differences in host ecology such as roost environments could also contribute to differential transmission. Additionally, we did not examine whether Red Knots shed AIV from the respiratory rather than the intestinal tract.
Laughing Gulls exhibited significant but variable increases in antibody prevalence over the stopover period; sample sizes were relatively small. Given that a majority of Ruddy Turnstones were exposed to AIV over the stopover season and that Laughing Gulls and turnstones share multiple habitats in the Delaware Bay area (data not shown), it is not surprising that many Laughing Gulls also were exposed. To date, Laughing Gulls have not been extensively sampled at Delaware Bay or elsewhere (reviewed by Bogomolni et al., 2008) and infection data are limited in this study. More systematic sampling of Laughing Gulls including time periods before shorebird arrival and after their departure would help to characterize AIV dynamics in this species further.
CONCLUSIONS
This study is the first to detail AIV infection dynamics over the entire course of an epidemic within Charadriiformes populations. The observed dynamical changes in prevalence and antibody prevalence allowed insight into when and where these four species were infected with or exposed to AIV. Although Ruddy Turnstones are seemingly the most important species in AIV epidemics at Delaware Bay, the detailed dynamic changes reported here indicate that they largely become infected, seroconvert, and recover locally. The source of viruses for these annual epidemics has not been identified, but AIV could arrive with a few infected Ruddy Turnstones (or other shorebirds) or be acquired from local sources such as gulls or waterfowl. Because infection apparently is a local phenomenon, Ruddy Turnstones might primarily act as a local virus amplifier, potentially generating reassortant viruses derived from many distant sources (Pearce et al., 2010). Other species, such as Red Knots, might be exposed at other (unidentified) sites, but further research is needed to define their possible roles in AIV epidemiology.
ACKNOWLEDGMENTS
This research was funded through Specific Cooperative Agreement 58-6612-2-0220 between the Southeast Poultry Research Laboratory, Agricultural Research Service, Department of Agriculture (USDA-ARS) and the Southeastern Cooperative Wildlife Disease Study, and by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH), Department of Health and Human Services, under contract HHSN266200700007C. The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Banding data and morphometric measurements are the property of the Natural Heritage & Endangered Species Program, Division of Fish and Wildlife, Delaware Department of Natural Resources and Environmental Control, and the Nongame and Endangered Species Program, Division of Fish and Wildlife, New Jersey Department of Environmental Protection; we thank these agencies for granting access to these data. We are grateful to many people who provided field and laboratory support, particularly E. Casey, L. Coffee, M. Cole, J. Cumbee, D. Downs, S. Gibbs, W. Hamrick, S. Keeler, G. Martin, S. McGraw, C. McKinnon, J. Murdock, R. Paulson, J. Smith, and B. Wilcox.
LITERATURE CITED
- BAKER AJ, GONZALEZ PM, PIERSMA T, NILES LJ, DO NASCIMENTO IDS, ATKINSON PW, CLARK NA, MINTON CDT, PECK MK, AND AARTS G. 2004. Rapid population decline in Red Knots: Fitness consequences of decreased refueling rates and late arrival in Delaware Bay. Proceedings of the Royal Society B: Biological Sciences 271: 875–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOGOMOLNI AL, GAST RJ, ELLIS JC, DENNETT M, PUGLIARES KR, LENTELL BJ, AND MOORE MJ. 2008. Victims or vectors: A survey of marine vertebrate zoonoses from coastal waters of the Northwest Atlantic. Diseases of Aquatic Organisms 81: 13–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN JD, STALLKNECHT DE, BERGHAUS RD, LUTTRELL MP, VELEK K, KISTLER W, COSTA T, YABSLEY MJ, AND SWAYNE D. 2009. Evaluation of a commercial blocking enzyme-linked immunosorbent assay to detect avian influenza virus antibodies in multiple experimentally infected avian species. Clinical and Vaccine Immunology 16: 824–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUEHLER DM, TIELEMAN BI, AND PIERSMA T. 2010. How do migratory species stay healthy over the annual cycle? A conceptual model for immune function and for resistance to disease. Integrative and Comparative Biology 50: 346–357. [DOI] [PubMed] [Google Scholar]
- BURGER J 1996. Laughing Gull (Larus atricilla). In The birds of North America online, Poole A (ed.). Cornell Lab of Ornithology, Ithaca, New York, Species Account no. 225, http://bna.birds.cornell.edu/bna/species/225. Accessed January 2011. [Google Scholar]
- BURGER J, CARLUCCI SA, JEITNER CW, AND NILES L. 2007. Habitat choice, disturbance, and management of foraging shorebirds and gulls at a migratory stopover. Journal of Coastal Research 23: 1159–1166. [Google Scholar]
- BURNHAM KP, AND ANDERSON DR. 2002. Model Selection and multimodel inference: A practical information-theoretic approach. 2nd Edition. Springer, New York, New York, 488 pp. [Google Scholar]
- CLARK KE, NILES LJ, AND BURGER J. 1993. Abundance and distribution of migrant shorebirds in Delaware Bay. Condor 95: 694–705. [Google Scholar]
- D’AMICO VL, BERTELLOTTI M, BAKER AJ, AND DIAZ LA. 2007. Exposure of Red Knots (Calidris canutus rufa) to select avian pathogens; Patagonia, Argentina. Journal of Wildlife Diseases 43: 794–797. [DOI] [PubMed] [Google Scholar]
- ESCUDERO G, MUNSTER VJ, BERTELLOTTI M, AND EDELAAR P. 2008. Perpetuation of avian influenza in the Americas: Examining the role of shorebirds in Patagonia. Auk 125: 494–495. [Google Scholar]
- FENTON A, AND PEDERSEN AB. 2005. Community epidemiology framework for classifying disease threats. Emerging Infectious Diseases 11: 1815–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOUCHIER RAM, MUNSTER V, WALLENSTEN A, BESTEBROER TM, HERFST S, SMITH D, RIMMELZWAAN GF, OLSEN B, AND OSTERHAUS ADME. 2005. Characterization of a novel influenza A virus hemagglutinin subtype (H16) obtained from Black-headed Gulls. Journal of Virology 79: 2814–2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GHERSI BM, BLAZES DL, ICOCHEA E, GONZALEZ RI, KOCHEL T, TINOCO Y, SOVERO MM, LINDSTROM S, SHU B, KLIMOV A, GONZALEZ AE, AND MONTGOMERY JM. 2009. Avian influenza in wild birds, central coast of Peru. Emerging Infectious Diseases 15: 935–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILLINGS S, ATKINSON PW, BARDSLEY SL, CLARK NA, LOVE SE, ROBINSON RA, STILLMAN RA, AND WEBER RG. 2007. Shorebird predation of horseshoe crab eggs in Delaware Bay: Species contrasts and availability constraints. Journal of Animal Ecology 76: 503–514. [DOI] [PubMed] [Google Scholar]
- GILLINGS S, ATKINSON PW, BAKER AJ, BENNETT KA, CLARK NA, COLE KB, GONZALEZ PM, KALASZ KS, MINTON CDT, NILES LJ, PORTER RC, DE LIMA SERRANO I, SITTERS HP, WOODS JL, AND SHAFFER TL. 2009. Staging behavior in Red Knot (Calidris canutus) in Delaware Bay: Implications for monitoring mass and population size. Auk 126: 54–63. [Google Scholar]
- HANSON BA, LUTTRELL MP, GOEKJIAN VH, NILES L, SWAYNE DE, SENNE DA, AND STALLKNECHT DE. 2008. Is the occurrence of avian influenza virus in Charadriiformes species and location dependent? Journal of Wildlife Diseases 44: 351–361. [DOI] [PubMed] [Google Scholar]
- HARRINGTON BA 2001. Red Knot (Calidris canutus). In The birds of North America online, Poole A (ed.). Cornell Lab of Ornithology, Ithaca, New York, Species Account no. 563, http://bna.birds.cornell.edu/bna/species/563. Accessed January 2011. [Google Scholar]
- HASTIE TJ, AND TIBSHIRANI RJ. 1990. Generalized additive models. Chapman and Hall, London, UK, 352 pp. [Google Scholar]
- HINSHAW VS, WEBSTER RG, AND TURNER B. 1979. Water-borne transmission of influenza A viruses. Intervirology 11: 66–68. [DOI] [PubMed] [Google Scholar]
- HINSHAW VS, AIR GM, SCHILD GC, AND NEWMAN RW. 1983. Characterization of a novel hemagglutinin subtype (H13) of influenza A viruses from gulls. Bulletin of the World Health Organization 61: 677–679. [PMC free article] [PubMed] [Google Scholar]
- HLINAK A, MUHLE RU, WERNER O, GLOBIG A, STARICK E, SCHIRRMEIER H, HOFFMANN B, ENGELHARDT A, HUBNER D, CONRATHS FJ, WALLSCHLAGER D, KRUCKENBERG H, AND MULLER T. 2006. A virological survey in migrating waders and other waterfowl in one of the most important resting sites of Germany. Journal of Veterinary Medicine Series B, Infectious Diseases and Veterinary Public Health 53: 105–110. [DOI] [PubMed] [Google Scholar]
- IVERSON SA, TAKEKAWA JY, SCHWARZBACH S, CARDONA CJ, WARNOCK N, BISHOP MA, SCHIRATO GA, PAROULEK S, ACKERMAN JT, IP H, AND BOYCE WM. 2008. Low prevalence of avian influenza virus in shorebirds on the Pacific coast of North America. Waterbirds 31: 602–610. [Google Scholar]
- JIN Y, YATES MV, THOMPSON SS, AND JURY WA. 1997. Sorption of viruses during flow through saturated sand columns. Environmental Science & Technology 31: 548–555. [Google Scholar]
- KAWAOKA Y, CHAMBERS TM, SLADEN WL, AND WEBSTER RG. 1988. Is the gene pool of influenza viruses in shorebirds and gulls different from that in wild ducks? Virology 163: 247–250. [DOI] [PubMed] [Google Scholar]
- KRAUSS S, WALKER D, PRYOR SP, NILES L, LI CH, HINSHAW VS, AND WEBSTER RG. 2004. Influenza A viruses of migrating wild aquatic birds in North America. Vector-Borne and Zoonotic Diseases 4: 177–189. [DOI] [PubMed] [Google Scholar]
- KRAUSS S, OBERT CA, FRANKS J, WALKER D, JONES K, SEILER P, NILES L, PRYOR SP, OBENAUER JC, NAEVE CW, WIDJAJA L, WEBBY RJ, AND WEBSTER RG. 2007. Influenza in migratory birds and evidence of limited inter-continental virus exchange. PLoS Pathogens 3: 1684–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRAUSS S, STALLKNECHT DE, NEGOVETICH NJ, NILES LJ, WEBBY RJ, AND WEBSTER RG. 2010. Coincident Ruddy Turnstone migration and horseshoe crab spawning creates an ecological “hot spot” for influenza viruses. Proceedings of the Royal Society B: Biological Sciences 277: 3373–3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LATORRE-MARGALEF N, GUNNARSSON G, MUNSTER VJ, FOUCHIER RAM, OSTERHAUS ADME, ELMBERG J, OLSEN B, WALLENSTEN A, HAEMIG PD, FRANSSON T, BRUDIN L, AND WALDENSTRÖM J. 2009. Effects of influenza A virus infection on migrating Mallard ducks. Proceedings of the Royal Society B: Biological Sciences 276: 1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LLOYD-SMITH JO, CROSS PC, BRIGGS CJ, DAUGHERTY M, GETZ WM, LATTO J, SANCHEZ MS, SMITH AB, AND SWEI A. 2005. Should we expect population thresholds for wildlife disease? Trends in Ecology & Evolution 20: 511–519. [DOI] [PubMed] [Google Scholar]
- MIZRAHI DS, HOLBERTON RL, AND GAUTHREAUX SA. 2001. Patterns of corticosterone secretion in migrating Semipalmated Sandpipers at a major spring stopover site. Auk 118: 79–91. [Google Scholar]
- MORRISON RIG, AND HARRINGTON BA. 1992. The migration system of the Red Knot Calidris canutus rufa in the New World. Wader Study Group Bulletin 64 (Supplement): 71–84. [Google Scholar]
- MORRISON RIG, GILL RE, HARRINGTON BA, SKAGEN S, PAGE GW, GRATTO-TREVOR CL, AND HAIG SM. 2001. Estimates of shorebird populations in North America. Canadian Wildlife Service Occasional Paper 104: 64. [Google Scholar]
- MUNSTER VJ, BAAS C, LEXMOND P, WALDENSTROM J, WALLENSTEN A, FRANSSON T, RIMMELZWAAN GF, BEYER WEP, SCHUTTEN M, Olsen B, Osterhaus A , and Fouchier RAM. 2007. Spatial, temporal, and species variation in prevalence of influenza A viruses in wild migratory birds. PLoS Pathogens 3: 630–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MYERS JP, SALLABERRY M, ORTIZ E, CASTRO G, GORDON LM, MARON JL, SCHICK CT, TABILO E, ANTAS P, AND BELOW T. 1990. Migration routes of New World Sanderlings (Calidris alba). Auk 107: 172–180. [Google Scholar]
- NETTLESHIP DN 2000. Ruddy Turnstone (Arenaria interpres). In The birds of North America online, Poole A (ed.). Cornell Lab of Ornithology, Ithaca, New York, Species Account no. 537, http://bna.birds.cornell.edu/bna/species/537. Accessed January 2011. [Google Scholar]
- NILES LJ, BART J, SITTERS HP, DEY AD, CLARK KE, ATKINSON PW, BAKER AJ, BENNETT KA, KALASZ KS, CLARK NA, CLARK J, GILLINGS S, GATES AS, GONZALEZ PM, HERNANDEZ DE, MINTON CDT, MORRISON RIG, PORTER RR, KEN ROSS R, AND VEITCH CR. 2009. Effects of horseshoe crab harvest in Delaware Bay on Red Knots: Are harvest restrictions working? Bioscience 59: 153–164. [Google Scholar]
- NUNN CL, AND ALTIZER S. 2006. Host–parasite dynamics and epidemiological principles. In Infectious diseases in primates: Behavior, ecology, and evolution, Nunn CL and Altizer S (eds.). Oxford University Press, New York, New York, pp. 98–133. [Google Scholar]
- OLSEN B, MUNSTER VJ, WALLENSTEN A, WALDENSTROM J, OSTERHAUS A, AND FOUCHIER RAM. 2006. Global patterns of influenza A virus in wild birds. Science 312: 384–388. [DOI] [PubMed] [Google Scholar]
- PEARCE JM, RAMEY AM, IP HS, AND GILL RE. 2010. Limited evidence of trans-hemispheric movement of avian influenza viruses among contemporary North American shorebird isolates. Virus Research 148: 44–50. [DOI] [PubMed] [Google Scholar]
- PIEROTTI RJ, AND GOOD TP. 1994. Herring Gull (Larus argentatus). In The birds of North America online, Poole A (ed.). Cornell Lab of Ornithology, Ithaca, New York, Species Account no. 124, http://bna.birds.cornell.edu/bna/species/124. Accessed January 2011. [Google Scholar]
- Piersma T, Reneerkens J , and Ramenofsky M. 2000. Baseline corticosterone peaks in shorebirds with maximal energy stores for migration: A general preparatory mechanism for rapid behavioral and metabolic transitions? General and Comparative Endocrinology 120: 118–126. [DOI] [PubMed] [Google Scholar]
- ROBINSON RA, ATKINSON PW, AND CLARK NA. 2003. Arrival and weight gain of Red Knot Calidris canutus, Ruddy Turnstone Arenaria interpres and Sanderling Calidris alba staging in Delaware Bay in spring. British Trust for Ornithology Research Report 307: 51. [Google Scholar]
- ROGAN WJ, AND GLADEN B. 1978. Estimating prevalence from the results of a screening test. American Journal of Epidemiology 107: 71–76. [DOI] [PubMed] [Google Scholar]
- SPACKMAN E 2009. The ecology of avian influenza virus in wild birds: What does this mean for poultry? Poultry Science 88: 847–850. [DOI] [PubMed] [Google Scholar]
- SPACKMAN E, AND SUAREZ D. 2008. Type A influenza virus detection and quantitation by real-time RT-PCR. Methods in Molecular Biology 436: 19–26. [DOI] [PubMed] [Google Scholar]
- STALLKNECHT DE, AND BROWN JD. 2007. Wild birds and the epidemiology of avian influenza. Journal of Wildlife Diseases 43: S15–S20. [DOI] [PubMed] [Google Scholar]
- SWAYNE DE, SENNE DA, AND BEARD CW. 1998. Avian influenza. In A laboratory manual for the isolation and identification of avian pathogens, Swayne DE (ed.). American Association of Avian Pathologists, Kennett Square, Pennsylvania, pp. 150–155. [Google Scholar]
- TSIPOURA N, AND BURGER J. 1999. Shorebird diet during spring migration stopover on Delaware Bay. Condor 101: 635–644. [Google Scholar]
- US FISH AND WILDLIFE SERVICE. 2003. Delaware Bay shorebird-horseshoe crab assessment report and peer review. US Fish and Wildlife Service Migratory Bird Publication; R9–03/02, Arlington, Virginia, 99 pp. [Google Scholar]
- VAHL WK, VAN DER MEER J, MEIJER K, PIERSMA T, AND WEISSING FJ. 2007. Interference competition, the spatial distribution of food and free-living foragers. Animal Behaviour 74: 1493–1503. [Google Scholar]
- VELARDE R, CALVIN SE, OJKIC D, BARKER IK, AND NAGY É. 2010. Avian influenza virus H13 circulating in Ring-billed Gulls (Larus delawarensis) in southern Ontario, Canada. Avian Diseases 54: 411–419. [DOI] [PubMed] [Google Scholar]
- WINKER K, MCCRACKEN KG, GIBSON DD, PRUETT CL, MEIER R, HUETTMANN F, WEGE M, KULIKOVA IV, ZHURAVLEV YN, PERDUE ML, SPACKMAN E, SUAREZ DL, AND SWAYNE DE. 2007. Movements of birds and avian influenza from Asia into Alaska. Emerging Infectious Diseases 13: 547–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINKER K, SPACKMAN E, AND SWAYNE DE. 2008. Rarity of influenza A virus in spring shorebirds, southern Alaska. Emerging Infectious Diseases 14: 1314–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMNIKOVA SS, GAMBARYAN AS, TUZIKOV AB, BOVIN NV, MATROSOVICH MN, FEDYAKINA IT, GRINEV AA, BLINOV VM, LVOV DK, SUAREZ DL, AND SWAYNE DE. 2003. Differences between HA receptor-binding sites of avian influenza viruses isolated from Laridae and Anatidae. Avian Diseases 47: 1164–1168. [DOI] [PubMed] [Google Scholar]