Abstract
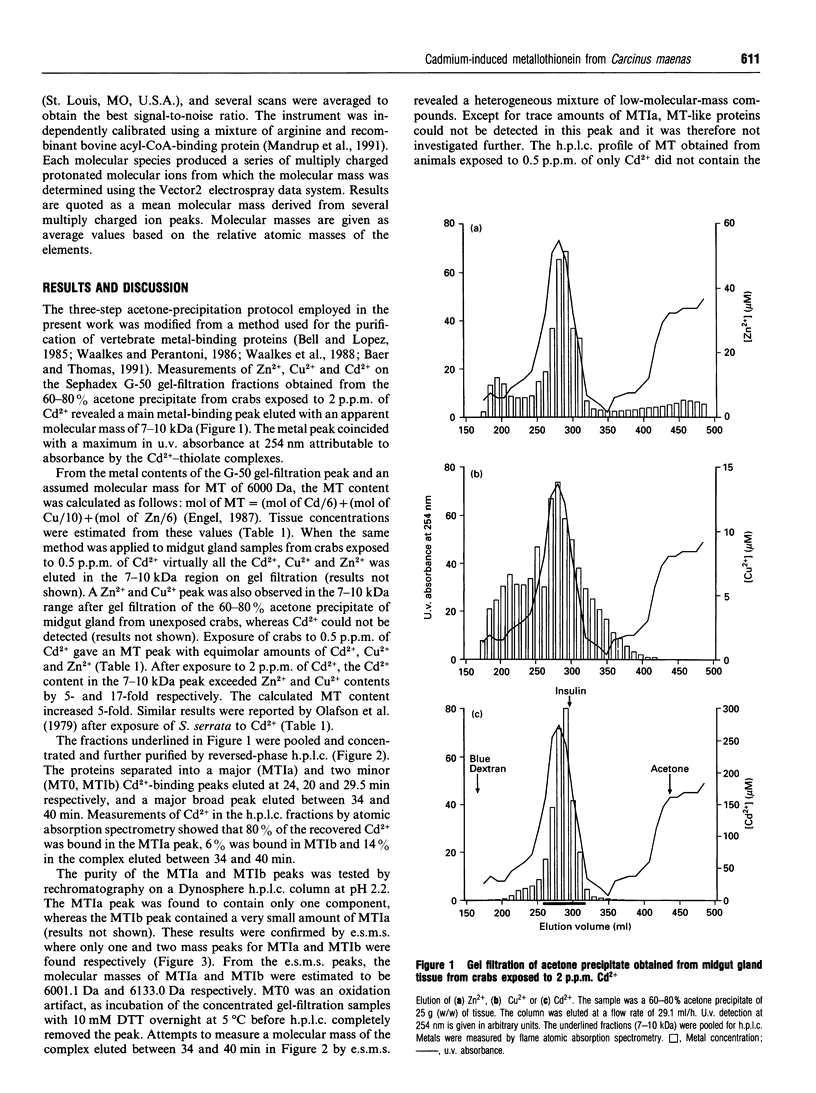
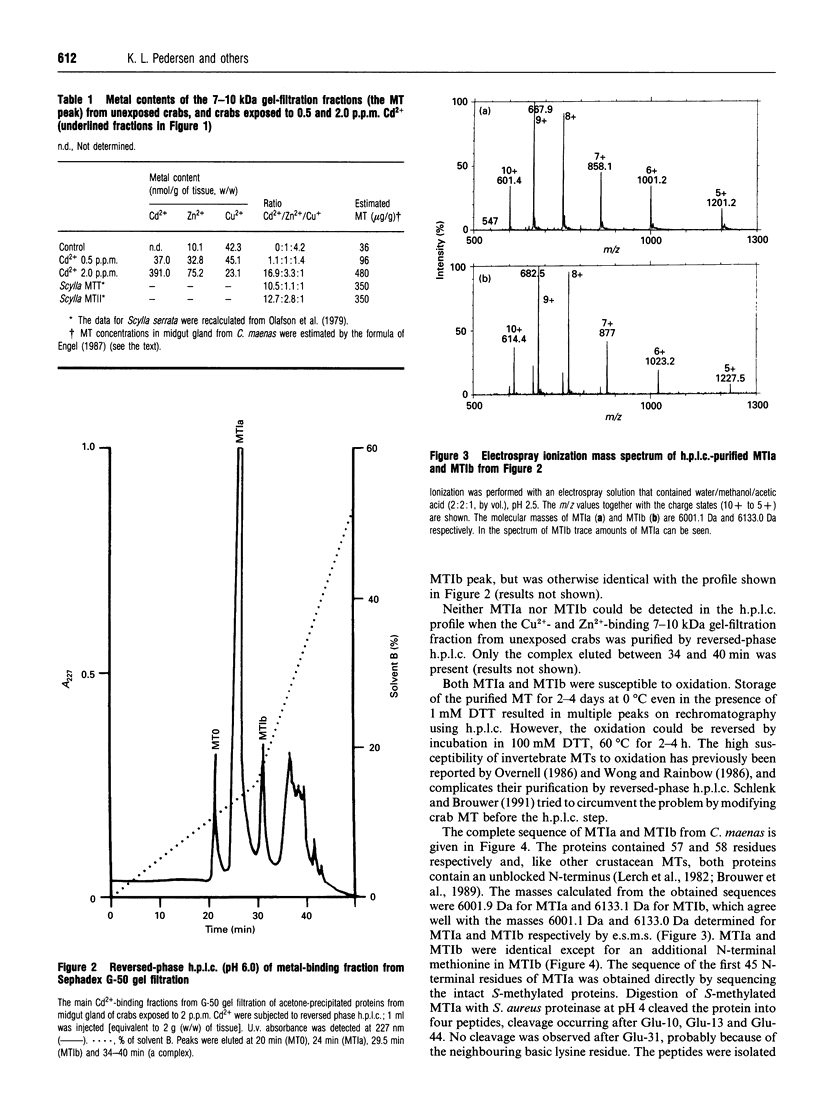
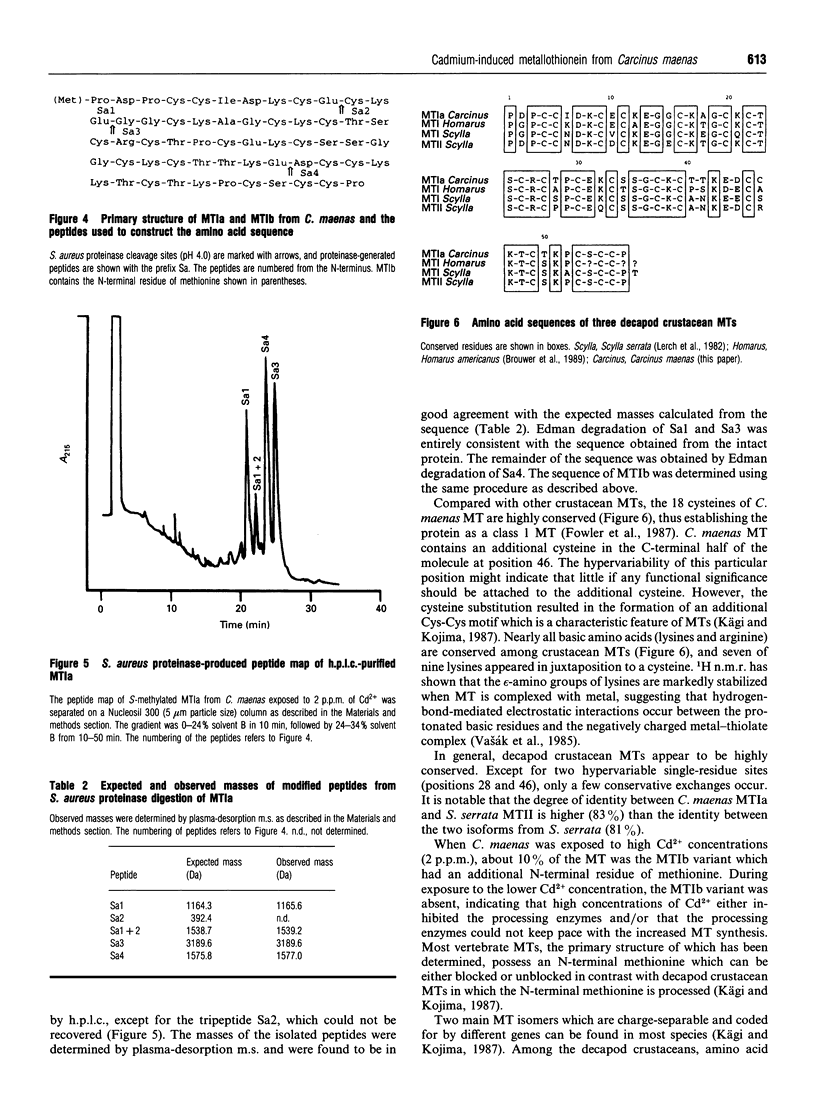
Two metallothionein variants were purified from the midgut gland of crabs (Carcinus maenas) exposed to a high cadmium concentration (2 p.p.m.). One of the variants was purified from crabs exposed to a low cadmium concentration (0.5 p.p.m.). The purification method involved acetone precipitation, gel filtration and reversed-phase h.p.l.c. The complete amino acid sequences of both variants have been elucidated by m.s. and automated sequence analysis on S-methylated proteins or fragments produced by cleavage of the S-methylated proteins with Staphylococcus aureus proteinase. The two variants from crabs exposed to the high cadmium concentration differed only by a single residue of methionine at the N-terminus. The single variant isolated from crabs exposed to the low cadmium concentration was the one without the N-terminal methionine, indicating that high cadmium concentrations either inhibit the processing enzymes and/or that the processing enzymes cannot keep pace with the increased metallothionein synthesis when cadmium availability is high. Cadmium-induced metallothionein from C. maenas shows a high degree of structural similarity to metallothioneins from the decapod crustaceans Scylla serrata and Homarus americanus.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe S., Matsumi M., Tsukioki M., Mizukawa S., Takahashi T., Iijima Y., Itano Y., Kosaka F. Metallothionein and zinc metabolism in endotoxin shock rats. Experientia Suppl. 1987;52:587–594. doi: 10.1007/978-3-0348-6784-9_61. [DOI] [PubMed] [Google Scholar]
- Bell J. U., Lopez J. M. Isolation and partial characterization of a cadmium-binding protein from the liver of alligators exposed to cadmium. Comp Biochem Physiol C. 1985;82(1):123–128. doi: 10.1016/0742-8413(85)90218-x. [DOI] [PubMed] [Google Scholar]
- Bernhard W. R., Vasák M., Kägi J. H. Cadmium binding and metal cluster formation in metallothionein: a differential modification study. Biochemistry. 1986 Apr 22;25(8):1975–1980. doi: 10.1021/bi00356a021. [DOI] [PubMed] [Google Scholar]
- Bernhard W. R., Vasák M., Kägi J. H. Cadmium binding and metal cluster formation in metallothionein: a differential modification study. Experientia Suppl. 1987;52:243–246. doi: 10.1007/978-3-0348-6784-9_18. [DOI] [PubMed] [Google Scholar]
- Briggs R. W., Armitage I. M. Evidence for site-selective metal binding in calf liver metallothionein. J Biol Chem. 1982 Feb 10;257(3):1259–1262. [PubMed] [Google Scholar]
- Brouwer M., Brouwer-Hoexum T. Interaction of copper-metallothionein from the American lobster, Homarus americanus, with glutathione. Arch Biochem Biophys. 1991 Oct;290(1):207–213. doi: 10.1016/0003-9861(91)90610-u. [DOI] [PubMed] [Google Scholar]
- Brouwer M., Schlenk D., Ringwood A. H., Brouwer-Hoexum T. Metal-specific induction of metallothionein isoforms in the blue crab Callinectes sapidus in response to single- and mixed-metal exposure. Arch Biochem Biophys. 1992 May 1;294(2):461–468. doi: 10.1016/0003-9861(92)90712-6. [DOI] [PubMed] [Google Scholar]
- Brouwer M., Winge D. R., Gray W. R. Structural and functional diversity of copper-metallothioneins from the American lobster Homarus americanus. J Inorg Biochem. 1989 Apr;35(4):289–303. doi: 10.1016/0162-0134(89)84018-8. [DOI] [PubMed] [Google Scholar]
- Cain K., Griffiths B. L. A comparison of isometallothionein synthesis in rat liver after partial hepatectomy and parenteral zinc injection. Biochem J. 1984 Jan 1;217(1):85–92. doi: 10.1042/bj2170085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstens E. B., Lu A. L., Chan H. L. Sequence, transcriptional mapping, and overexpression of p47, a baculovirus gene regulating late gene expression. J Virol. 1993 May;67(5):2513–2520. doi: 10.1128/jvi.67.5.2513-2520.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casterline J. L., Jr, Yip G. The distribution and binding of cadmium in oyster, soybean, and rat liver and kidney. Arch Environ Contam Toxicol. 1975;3(3):319–329. doi: 10.1007/BF02220744. [DOI] [PubMed] [Google Scholar]
- Foster R., Gedamu L. Functional analyses of promoter elements responsible for the differential expression of the human metallothionein (MT)-IG and MT-IF genes. J Biol Chem. 1991 May 25;266(15):9866–9875. [PubMed] [Google Scholar]
- Fowler B. A., Hildebrand C. E., Kojima Y., Webb M. Nomenclature of metallothionein. Experientia Suppl. 1987;52:19–22. doi: 10.1007/978-3-0348-6784-9_2. [DOI] [PubMed] [Google Scholar]
- Hamer D. H. Metallothionein. Annu Rev Biochem. 1986;55:913–951. doi: 10.1146/annurev.bi.55.070186.004405. [DOI] [PubMed] [Google Scholar]
- Hunziker P. E. Cysteine modification of metallothionein. Methods Enzymol. 1991;205:399–400. doi: 10.1016/0076-6879(91)05121-b. [DOI] [PubMed] [Google Scholar]
- Hunziker P. E. Metal removal from mammalian metallothioneins. Methods Enzymol. 1991;205:451–452. doi: 10.1016/0076-6879(91)05129-j. [DOI] [PubMed] [Google Scholar]
- Jahroudi N., Foster R., Price-Haughey J., Beitel G., Gedamu L. Cell-type specific and differential regulation of the human metallothionein genes. Correlation with DNA methylation and chromatin structure. J Biol Chem. 1990 Apr 15;265(11):6506–6511. [PubMed] [Google Scholar]
- Klaassen C. D., Lehman-McKeeman L. D. Regulation of the isoforms of metallothionein. Biol Trace Elem Res. 1989 Jul-Sep;21:119–129. doi: 10.1007/BF02917244. [DOI] [PubMed] [Google Scholar]
- Kägi J. H., Kojima Y. Chemistry and biochemistry of metallothionein. Experientia Suppl. 1987;52:25–61. doi: 10.1007/978-3-0348-6784-9_3. [DOI] [PubMed] [Google Scholar]
- Lerch K., Ammer D., Olafson R. W. Crab metallothionein. Primary structures of metallothioneins 1 and 2. J Biol Chem. 1982 Mar 10;257(5):2420–2426. [PubMed] [Google Scholar]
- Lin L. Y., Lin W. C., Huang P. C. Pigeon metallothionein consists of two species. Biochim Biophys Acta. 1990 Feb 9;1037(2):248–255. doi: 10.1016/0167-4838(90)90175-f. [DOI] [PubMed] [Google Scholar]
- Lin L. Y., Liu L. F., Tam M. F., Huang P. C., Vestling M., Fenselau C. Primary sequence of duck metallothionein. Biochim Biophys Acta. 1990 Oct 18;1041(1):31–35. doi: 10.1016/0167-4838(90)90118-y. [DOI] [PubMed] [Google Scholar]
- Mandrup S., Højrup P., Kristiansen K., Knudsen J. Gene synthesis, expression in Escherichia coli, purification and characterization of the recombinant bovine acyl-CoA-binding protein. Biochem J. 1991 Jun 15;276(Pt 3):817–823. doi: 10.1042/bj2760817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick C. C., Fullmer C. S., Garvey J. S. Amino acid sequence and comparative antigenicity of chicken metallothionein. Proc Natl Acad Sci U S A. 1988 Jan;85(2):309–313. doi: 10.1073/pnas.85.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra R. K., Bremner I. Studies on the metabolism of rat liver copper-metallothionein. Biochem J. 1985 May 1;227(3):903–908. doi: 10.1042/bj2270903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath R., Kambadur R., Gulati S., Paliwal V. K., Sharma M. Molecular aspects, physiological function, and clinical significance of metallothioneins. Crit Rev Food Sci Nutr. 1988;27(1):41–85. doi: 10.1080/10408398809527477. [DOI] [PubMed] [Google Scholar]
- Nomiyama K., Nomiyama H. High-performance liquid chromatographic determination of tissue metallothionein in monkeys chronically exposed to cadmium. J Chromatogr. 1982 Mar 12;228:285–291. doi: 10.1016/s0378-4347(00)80442-9. [DOI] [PubMed] [Google Scholar]
- Otvos J. D., Olafson R. W., Armitage I. M. Structure of an invertebrate metallothionein from Scylla serrata. J Biol Chem. 1982 Mar 10;257(5):2427–2431. [PubMed] [Google Scholar]
- Overnell J. Occurrence of cadmium in crabs (Cancer pagurus) and the isolation and properties of cadmium metallothionein. Environ Health Perspect. 1986 Mar;65:101–105. doi: 10.1289/ehp.8665101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards R. I., Heguy A., Karin M. Structural and functional analysis of the human metallothionein-IA gene: differential induction by metal ions and glucocorticoids. Cell. 1984 May;37(1):263–272. doi: 10.1016/0092-8674(84)90322-2. [DOI] [PubMed] [Google Scholar]
- Sadhu C., Gedamu L. Regulation of human metallothionein (MT) genes. Differential expression of MTI-F, MTI-G, and MTII-A genes in the hepatoblastoma cell line (HepG2). J Biol Chem. 1988 Feb 25;263(6):2679–2684. [PubMed] [Google Scholar]
- Suzuki K. T., Ebihara Y., Akitomi H., Nishikawa M., Kawamura R. Change in ratio of the two hepatic isometallothioneins with development from prenatal to neonatal rats. Comp Biochem Physiol C. 1983;76(1):33–38. doi: 10.1016/0742-8413(83)90040-3. [DOI] [PubMed] [Google Scholar]
- Templeton D. M., Cherian M. G. Toxicological significance of metallothionein. Methods Enzymol. 1991;205:11–24. doi: 10.1016/0076-6879(91)05079-b. [DOI] [PubMed] [Google Scholar]
- Waalkes M. P., Perantoni A. Isolation of a novel metal-binding protein from rat testes. Characterization and distinction from metallothionein. J Biol Chem. 1986 Oct 5;261(28):13097–13103. [PubMed] [Google Scholar]
- Waalkes M. P., Perantoni A., Palmer A. E. Isolation and partial characterization of the low-molecular-mass zinc/cadmium-binding protein from the testes of the patas monkey (Erythrocebus patas). Distinction from metallothionein. Biochem J. 1988 Nov 15;256(1):131–137. doi: 10.1042/bj2560131. [DOI] [PMC free article] [PubMed] [Google Scholar]


