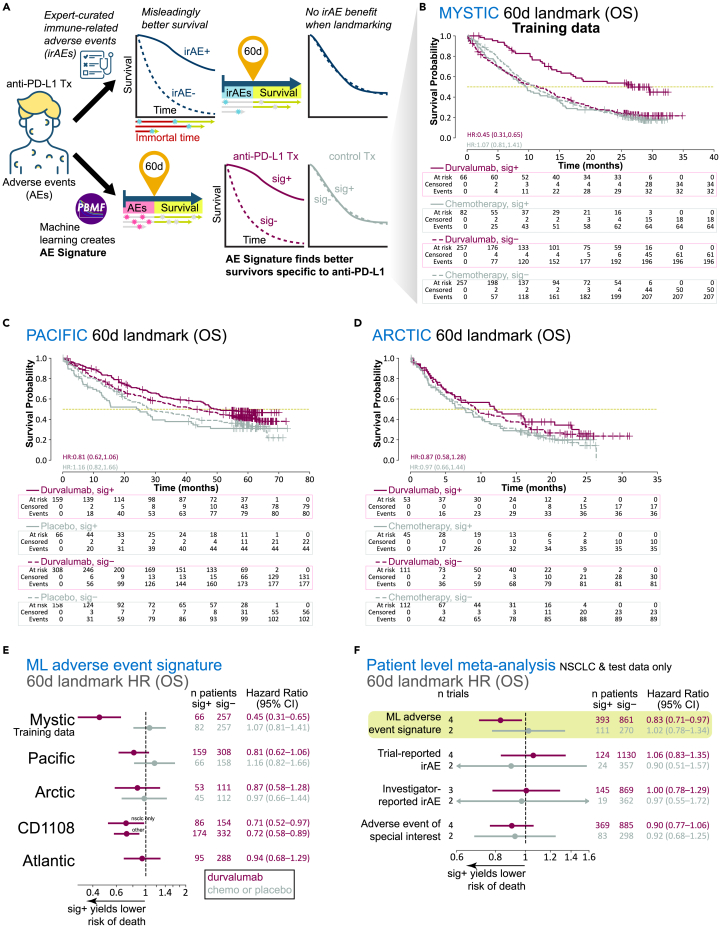
Figure 1.
Generation and evaluation of a machine learning-derived adverse event signature (AE signature)
(A) Analysis workflow diagram contrasting the use of irAEs versus the AE signature derived with our machine learning framework, the PBMF.
(B) Kaplan-Meier plots for OS, per treatment arm and signature class, for MYSTIC trial data landmarked at 60 days.
(C) Kaplan-Meier plots for OS, per treatment arm and signature class, for PACIFIC trial data landmarked at 60 days.
(D) Kaplan-Meier plots for OS, per treatment arm and signature class, for ARCTIC trial data landmarked at 60 days.
(E) Point estimates and 95% confidence intervals for hazard ratios (Cox proportional hazards regression model) with machine learning (ML) AE signature class as the only covariate, computed per treatment arm, per trial, after AE and OS data were landmarked at 60 days.
(F) Point estimates and 95% confidence intervals for hazard ratios (patient-level meta-analysis; Cox proportional hazards regression model with random effects for trial, indication, and treatment; fixed effects for signature class, age, sex, race, geographical region, tumor stage, and histology), after AE and OS data were landmarked at 60 days (excluding training data), and by signature or one of the three different definitions of an irAE. Chemo, chemotherapy; HR, hazard ratio; sig, ML AE signature.