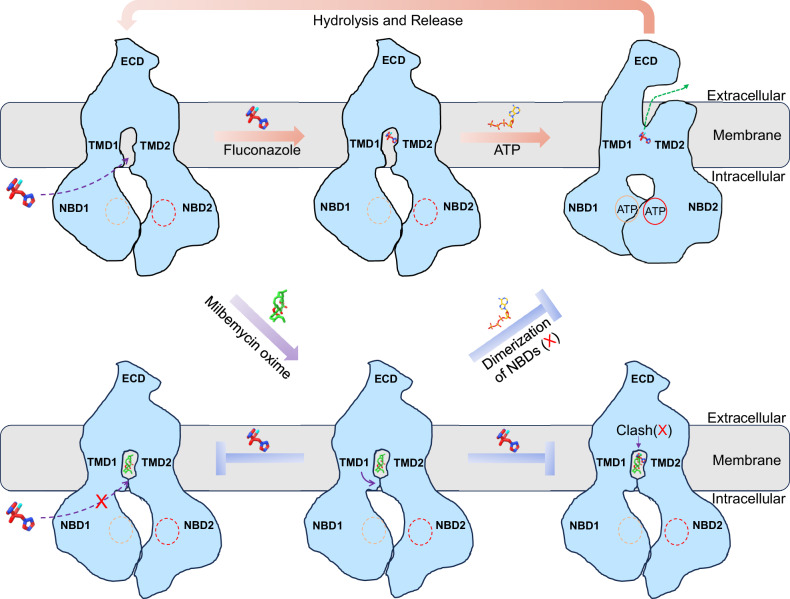
Fig. 6. Model for fluconazole efflux and inhibition by milbemycin oxime.
In the resting state, Cdr1 maintains an inward-open conformation, allowing the entry of hydrophilic fluconazole from the cytoplasm (dash purple arrow). As fluconazole enters the hydrophobic cavity, ATP molecules act as molecular glue to induce dimerization of NBDs and trigger a conformational change from inward to outward, leading to the release of fluconazole (dash green arrow). Following the hydrolysis of the ATP in NBD2, Cdr1 returns to its inward-facing conformation, starting the next cycle of fluconazole transport. Milbemycin oxime, upon entering the hydrophobic cavity, induces a conformational change that closes the entrances (purple arrow). It competes for binding at the central hydrophobic cavity, obstructing fluconazole. Additionally, the closure of the entrances effectively blocks the entry of fluconazole into this region (blocked dash purple arrow). Furthermore, milbemycin oxime acts as a molecular glue, locking the two TMDs, inhibiting ATPase activity, and preventing the transportation process.